730. Why is water used to cool some mechanisms?
Water has a high specific heat capacity, which contributes to good heat removal from the mechanism.
731. In what case should more energy be expended: for heating one liter of water by 1 °C or for heating one hundred grams of water by 1 °C?
To heat a liter of water, since the larger the mass, the more energy needs to be expended.
732. Cupronickel and silver forks of the same mass were dipped into hot water. Do they receive the same amount of heat from water?
A cupronickel fork will receive more heat, because the specific heat of cupronickel is greater than that of silver.
733. A piece of lead and a piece of cast iron of the same mass were hit three times with a sledgehammer. Which part got hotter?
Lead will heat up more because its specific heat capacity is less than cast iron, and less energy is needed to heat the lead.
734. One flask contains water, the other contains kerosene of the same mass and temperature. An equally heated iron cube was thrown into each flask. What will heat up to a higher temperature - water or kerosene?
Kerosene.
735. Why are temperature fluctuations less sharp in winter and summer in cities on the seashore than in cities located inland?
Water heats up and cools down more slowly than air. In winter, it cools down and moves warm air masses on land, making the climate on the coast warmer.
736. The specific heat capacity of aluminum is 920 J/kg °C. What does this mean?
This means that it takes 920 J to heat 1 kg of aluminum by 1 °C.
737. Aluminum and copper bars of the same mass of 1 kg are cooled by 1 °C. How much will the internal energy of each block change? Which bar will change more and by how much?
738. What amount of heat is needed to heat a kilogram iron billet by 45 °C?

739. How much heat is required to heat 0.25 kg of water from 30°C to 50°C?

740. How will the internal energy of two liters of water change when heated by 5 °C?

741. How much heat is needed to heat 5 g of water from 20 °C to 30 °C?
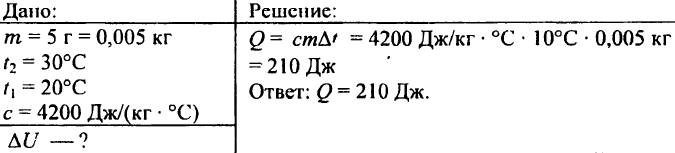
742. What amount of heat is needed to heat an aluminum ball weighing 0.03 kg by 72 °C?

743. Calculate the amount of heat required to heat 15 kg of copper by 80 °C.
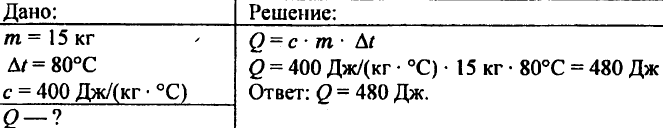
744. Calculate the amount of heat required to heat 5 kg of copper from 10 °C to 200 °C.

745. What amount of heat is required to heat 0.2 kg of water from 15 °C to 20 °C?

746. Water weighing 0.3 kg has cooled down by 20 °C. By how much is the internal energy of water reduced?

747. How much heat is needed to heat 0.4 kg of water at a temperature of 20 °C to a temperature of 30 °C?

748. How much heat is spent on heating 2.5 kg of water by 20 °C?
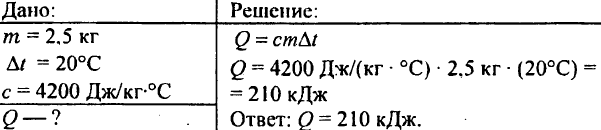
749. How much heat was released when 250 g of water cooled from 90 °C to 40 °C?
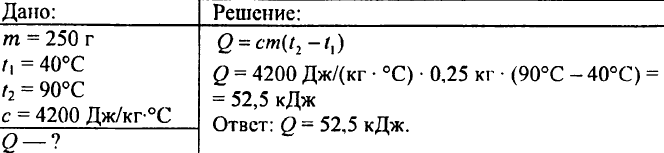
750. What amount of heat is required to heat 0.015 liters of water by 1 °C?

751. Calculate the amount of heat required to heat a pond with a volume of 300 m3 by 10 °C?
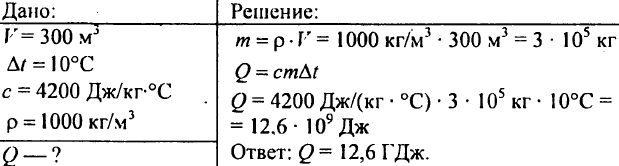
752. How much heat must be imparted to 1 kg of water in order to raise its temperature from 30°C to 40°C?

753. Water with a volume of 10 liters has cooled down from a temperature of 100 °C to a temperature of 40 °C. How much heat is released in this case?

754. Calculate the amount of heat required to heat 1 m3 of sand by 60 °C.

755. Air volume 60 m3, specific heat capacity 1000 J/kg °C, air density 1.29 kg/m3. How much heat is needed to raise it to 22°C?

756. Water was heated by 10 ° C, spending 4.20 103 J of heat. Determine the amount of water.
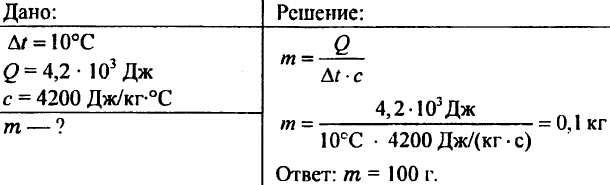
757. Water weighing 0.5 kg reported 20.95 kJ of heat. What was the temperature of the water if the initial temperature of the water was 20°C?

758. 8 kg of water at 10 °C is poured into a copper saucepan weighing 2.5 kg. How much heat is needed to bring the water to a boil in a saucepan?

759. A liter of water at a temperature of 15 °C is poured into a copper ladle weighing 300 g. How much heat is needed to heat the water in the ladle by 85 °C?

760. A piece of heated granite weighing 3 kg is placed in water. Granite transfers 12.6 kJ of heat to water, cooling by 10 °C. What is the specific heat capacity of the stone?

761. Hot water at 50°C was added to 5 kg of water at 12°C, obtaining a mixture with a temperature of 30°C. How much water was added?

762. Water at 20°C was added to 3 liters of water at 60°C to obtain water at 40°C. How much water was added?

763. What will be the temperature of the mixture if 600 g of water at 80 °C are mixed with 200 g of water at 20 °C?

764. A liter of water at 90°C was poured into water at 10°C, and the temperature of the water became 60°C. How much cold water was there?

765. Determine how much hot water heated to 60°C should be poured into a vessel if the vessel already contains 20 liters of cold water at a temperature of 15°C; the temperature of the mixture should be 40 °C.

766. Determine how much heat is required to heat 425 g of water by 20 °C.

767. How many degrees will 5 kg of water heat up if the water receives 167.2 kJ?

768. How much heat is required to heat m grams of water at a temperature t1 to a temperature t2?
769. 2 kg of water is poured into a calorimeter at a temperature of 15 °C. To what temperature will the water of the calorimeter heat up if a brass weight of 500 g heated to 100 °C is lowered into it? The specific heat capacity of brass is 0.37 kJ/(kg °C).
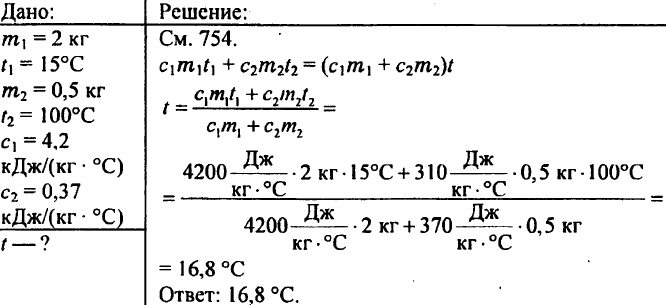
770. There are pieces of copper, tin and aluminum of the same volume. Which of these pieces has the largest and which the smallest heat capacity?

771. 450 g of water, the temperature of which is 20 °C, was poured into the calorimeter. When 200 g of iron filings heated to 100°C were immersed in this water, the temperature of the water became 24°C. Determine the specific heat capacity of sawdust.

772. A copper calorimeter weighing 100 g holds 738 g of water, the temperature of which is 15 °C. 200 g of copper was lowered into this calorimeter at a temperature of 100 °C, after which the temperature of the calorimeter rose to 17 °C. What is the specific heat capacity of copper?

773. A steel ball weighing 10 g is taken out of the furnace and lowered into water at a temperature of 10 °C. The water temperature rose to 25°C. What was the temperature of the ball in the oven if the mass of water is 50 g? The specific heat capacity of steel is 0.5 kJ/(kg °C).

777. 50 g of water at 19 °C are poured into water weighing 150 g at a temperature of 35 °C. What is the temperature of the mixture?

778. Water weighing 5 kg at 90 °C was poured into a cast-iron kettle weighing 2 kg at a temperature of 10 °C. What was the temperature of the water?

779. A steel chisel weighing 2 kg was heated to a temperature of 800 °C and then lowered into a vessel containing 15 liters of water at a temperature of 10 °C. To what temperature will the water in the vessel be heated?
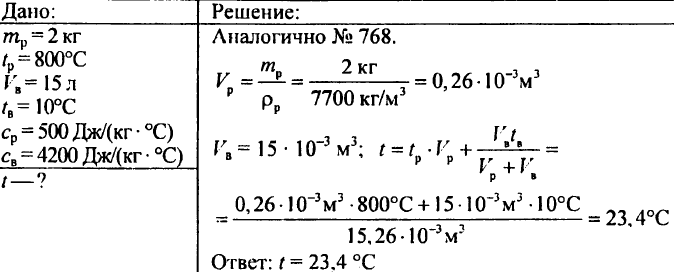
(Indication. To solve this problem, it is necessary to create an equation in which the desired temperature of the water in the vessel after the cutter is lowered is taken as the unknown.)
780. What temperature will water get if you mix 0.02 kg of water at 15 °C, 0.03 kg of water at 25 °C, and 0.01 kg of water at 60 °C?
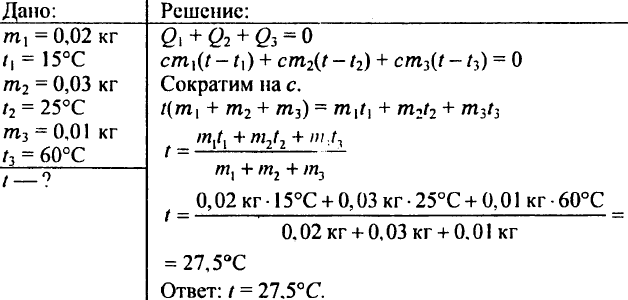
781. Heating a well-ventilated class requires an amount of heat of 4.19 MJ per hour. Water enters the heating radiators at 80°C and exits at 72°C. How much water should be supplied to the radiators every hour?

782. Lead weighing 0.1 kg at a temperature of 100 °C was immersed in an aluminum calorimeter weighing 0.04 kg containing 0.24 kg of water at a temperature of 15 °C. After that, the temperature of 16 °C was established in the calorimeter. What is the specific heat capacity of lead?

What heats up faster on the stove - a kettle or a bucket of water? The answer is obvious - a kettle. Then the second question is why?
The answer is no less obvious - because the mass of water in the kettle is less. Fine. And now you can do the most real physical experience yourself at home. To do this, you will need two identical small saucepans, an equal amount of water and vegetable oil, for example, half a liter each and a stove. Put pots of oil and water on the same fire. And now just watch what will heat up faster. If there is a thermometer for liquids, you can use it, if not, you can just try the temperature from time to time with your finger, just be careful not to burn yourself. In any case, you will soon see that the oil heats up significantly faster than water. And one more question, which can also be implemented in the form of experience. Which boils faster - warm water or cold? Everything is obvious again - the warm one will be the first to finish. Why all these strange questions and experiments? In order to determine the physical quantity called "the amount of heat."
Quantity of heat
The amount of heat is the energy that the body loses or gains during heat transfer. This is clear from the name. When cooling, the body will lose a certain amount of heat, and when heated, it will absorb. And the answers to our questions showed us what does the amount of heat depend on? First, the greater the mass of the body, the greater the amount of heat that must be expended to change its temperature by one degree. Secondly, the amount of heat necessary to heat a body depends on the substance of which it is composed, that is, on the kind of substance. And thirdly, the difference in body temperature before and after heat transfer is also important for our calculations. Based on the foregoing, we can determine the amount of heat by the formula:
Q=cm(t_2-t_1) ,
where Q is the amount of heat,
m - body weight,
(t_2-t_1) - the difference between the initial and final body temperatures,
c - specific heat capacity of the substance, is found from the relevant tables.
Using this formula, you can calculate the amount of heat that is necessary to heat any body or that this body will release when it cools.
The amount of heat is measured in joules (1 J), like any other form of energy. However, this value was introduced not so long ago, and people began to measure the amount of heat much earlier. And they used a unit that is widely used in our time - a calorie (1 cal). 1 calorie is the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius. Guided by these data, lovers of counting calories in the food they eat can, for the sake of interest, calculate how many liters of water can be boiled with the energy that they consume with food during the day.
The change in internal energy by doing work is characterized by the amount of work, i.e. work is a measure of the change in internal energy in a given process. The change in the internal energy of a body during heat transfer is characterized by a quantity called the amount of heat.
is the change in the internal energy of the body in the process of heat transfer without doing work. The amount of heat is denoted by the letter Q .
Work, internal energy and the amount of heat are measured in the same units - joules ( J), like any other form of energy.
In thermal measurements, a special unit of energy, the calorie ( feces), equal to the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius (more precisely, from 19.5 to 20.5 ° C). This unit, in particular, is currently used in calculating the consumption of heat (thermal energy) in apartment buildings. Empirically, the mechanical equivalent of heat has been established - the ratio between calories and joules: 1 cal = 4.2 J.

When a body transfers a certain amount of heat without doing work, its internal energy increases, if a body gives off a certain amount of heat, then its internal energy decreases.
If you pour 100 g of water into two identical vessels, and 400 g into another at the same temperature and put them on the same burners, then the water in the first vessel will boil earlier. Thus, the greater the mass of the body, the greater the amount of heat it needs to heat up. The same goes for cooling.

The amount of heat required to heat a body also depends on the kind of substance from which this body is made. This dependence of the amount of heat required to heat the body on the type of substance is characterized by a physical quantity called specific heat capacity substances.
- this is a physical quantity equal to the amount of heat that must be reported to 1 kg of a substance to heat it by 1 ° C (or 1 K). The same amount of heat is given off by 1 kg of a substance when cooled by 1 °C.
The specific heat capacity is denoted by the letter with. The unit of specific heat capacity is 1 J/kg °C or 1 J/kg °K.
The values of the specific heat capacity of substances are determined experimentally. Liquids have a higher specific heat capacity than metals; Water has the highest specific heat capacity, gold has a very small specific heat capacity.
Since the amount of heat is equal to the change in the internal energy of the body, we can say that the specific heat capacity shows how much the internal energy changes 1 kg substance when its temperature changes 1 °C. In particular, the internal energy of 1 kg of lead, when it is heated by 1 °C, increases by 140 J, and when it is cooled, it decreases by 140 J.
Q required to heat the body mass m temperature t 1 °С up to temperature t 2 °С, is equal to the product of the specific heat capacity of the substance, body mass and the difference between the final and initial temperatures, i.e.Q \u003d c ∙ m (t 2 - t 1)
According to the same formula, the amount of heat that the body gives off when cooled is also calculated. Only in this case should the final temperature be subtracted from the initial temperature, i.e. Subtract the smaller temperature from the larger temperature.

This is a synopsis on the topic. "Quantity of heat. Specific heat". Choose next steps:
- Go to the next abstract:
« Physics - Grade 10 "
In what processes does aggregate transformation of matter occur?
How can the state of matter be changed?
You can change the internal energy of any body by doing work, heating or, conversely, cooling it.
Thus, when forging a metal, work is done and it is heated, while at the same time the metal can be heated over a burning flame.
Also, if the piston is fixed (Fig. 13.5), then the volume of gas does not change when heated and no work is done. But the temperature of the gas, and hence its internal energy, increases.
Internal energy can increase and decrease, so the amount of heat can be positive or negative.
The process of transferring energy from one body to another without doing work is called heat exchange.
The quantitative measure of the change in internal energy during heat transfer is called amount of heat.
Molecular picture of heat transfer.
During heat exchange at the boundary between bodies, slowly moving molecules of a cold body interact with rapidly moving molecules of a hot body. As a result, the kinetic energies of the molecules are equalized and the velocities of the molecules of a cold body increase, while those of a hot body decrease.
During heat exchange, there is no conversion of energy from one form to another; part of the internal energy of a hotter body is transferred to a less heated body.
The amount of heat and heat capacity.
You already know that in order to heat a body with mass m from temperature t 1 to temperature t 2, it is necessary to transfer to it the amount of heat:
Q \u003d cm (t 2 - t 1) \u003d cm Δt. (13.5)
When the body cools, its final temperature t 2 turns out to be less than the initial temperature t 1 and the amount of heat given off by the body is negative.
The coefficient c in formula (13.5) is called specific heat capacity substances.
Specific heat- this is a value numerically equal to the amount of heat that a substance with a mass of 1 kg receives or gives off when its temperature changes by 1 K.
The specific heat capacity of gases depends on the process by which heat is transferred. If you heat a gas at constant pressure, it will expand and do work. To heat a gas by 1 °C at constant pressure, it needs to transfer more heat than to heat it at a constant volume, when the gas will only heat up.
Liquids and solids expand slightly when heated. Their specific heat capacities at constant volume and constant pressure differ little.
Specific heat of vaporization.
To convert a liquid into vapor during the boiling process, it is necessary to transfer a certain amount of heat to it. The temperature of a liquid does not change when it boils. The transformation of liquid into vapor at a constant temperature does not lead to an increase in the kinetic energy of molecules, but is accompanied by an increase in the potential energy of their interaction. After all, the average distance between gas molecules is much greater than between liquid molecules.
The value numerically equal to the amount of heat required to convert a 1 kg liquid into steam at a constant temperature is called specific heat of vaporization.
The process of liquid evaporation occurs at any temperature, while the fastest molecules leave the liquid, and it cools during evaporation. The specific heat of vaporization is equal to the specific heat of vaporization.
This value is denoted by the letter r and is expressed in joules per kilogram (J / kg).
The specific heat of vaporization of water is very high: r H20 = 2.256 10 6 J/kg at a temperature of 100 °C. In other liquids, such as alcohol, ether, mercury, kerosene, the specific heat of vaporization is 3-10 times less than that of water.
To convert a liquid of mass m into steam, an amount of heat is required equal to:
Q p \u003d rm. (13.6)
When steam condenses, the same amount of heat is released:
Q k \u003d -rm. (13.7)
Specific heat of fusion.
When a crystalline body melts, all the heat supplied to it goes to increase the potential energy of interaction of molecules. The kinetic energy of the molecules does not change, since melting occurs at a constant temperature.
The value numerically equal to the amount of heat required to transform a crystalline substance weighing 1 kg at a melting point into a liquid is called specific heat of fusion and are denoted by the letter λ.
During the crystallization of a substance with a mass of 1 kg, exactly the same amount of heat is released as is absorbed during melting.
The specific heat of melting of ice is rather high: 3.34 10 5 J/kg.
“If ice did not have a high heat of fusion, then in spring the entire mass of ice would have to melt in a few minutes or seconds, since heat is continuously transferred to ice from the air. The consequences of this would be dire; for even under the present situation great floods and great torrents of water arise from the melting of great masses of ice or snow.” R. Black, 18th century
In order to melt a crystalline body of mass m, an amount of heat is required equal to:
Qpl \u003d λm. (13.8)
The amount of heat released during the crystallization of the body is equal to:
Q cr = -λm (13.9)
Heat balance equation.
Consider heat exchange within a system consisting of several bodies initially having different temperatures, for example, heat exchange between water in a vessel and a hot iron ball lowered into water. According to the law of conservation of energy, the amount of heat given off by one body is numerically equal to the amount of heat received by another.
The given amount of heat is considered negative, the received amount of heat is considered positive. Therefore, the total amount of heat Q1 + Q2 = 0.
If heat exchange occurs between several bodies in an isolated system, then
Q 1 + Q 2 + Q 3 + ... = 0. (13.10)
Equation (13.10) is called heat balance equation.
Here Q 1 Q 2 , Q 3 - the amount of heat received or given away by the bodies. These quantities of heat are expressed by formula (13.5) or formulas (13.6) - (13.9), if various phase transformations of the substance occur in the process of heat transfer (melting, crystallization, vaporization, condensation).
You can change the internal energy of the gas in the cylinder not only by doing work, but also by heating the gas (Fig. 43). If the piston is fixed, then the volume of the gas will not change, but the temperature, and hence the internal energy, will increase.
The process of transferring energy from one body to another without doing work is called heat transfer or heat transfer.
The energy transferred to the body as a result of heat transfer is called the amount of heat. The amount of heat is also called the energy that the body gives off in the process of heat transfer.
Molecular picture of heat transfer. During heat exchange at the boundary between bodies, the slowly moving molecules of a cold body interact with the faster moving molecules of a hot body. As a result, the kinetic energies
molecules are aligned and the velocities of the molecules of a cold body increase, and those of a hot one decrease.
During heat exchange, there is no conversion of energy from one form to another: part of the internal energy of a hot body is transferred to a cold body.
The amount of heat and heat capacity. From the class VII physics course, it is known that in order to heat a body with a mass from temperature to temperature, it is necessary to inform it of the amount of heat
![]()
When the body cools, its final temperature is less than the initial one and the amount of heat given off by the body is negative.
The coefficient c in formula (4.5) is called the specific heat capacity. Specific heat capacity is the amount of heat that 1 kg of a substance receives or gives off when its temperature changes by 1 K -
Specific heat capacity is expressed in joules per kilogram times kelvin. Different bodies require an unequal amount of energy to increase the temperature by I K. Thus, the specific heat capacity of water and copper
The specific heat capacity depends not only on the properties of the substance, but also on the process by which heat transfer takes place. If you heat a gas at constant pressure, it will expand and do work. To heat a gas by 1 °C at constant pressure, it will need to transfer more heat than to heat it at constant volume.
Liquids and solids expand slightly when heated, and their specific heat capacities at constant volume and constant pressure differ little.
Specific heat of vaporization. To convert a liquid into vapor, a certain amount of heat must be transferred to it. The temperature of the liquid does not change during this transformation. The transformation of a liquid into vapor at a constant temperature does not lead to an increase in the kinetic energy of the molecules, but is accompanied by an increase in their potential energy. After all, the average distance between gas molecules is many times greater than between liquid molecules. In addition, an increase in volume during the transition of a substance from a liquid to a gaseous state requires work to be performed against the forces of external pressure.
The amount of heat required to turn 1 kg of liquid into vapor at a constant temperature is called
specific heat of vaporization. This value is denoted by a letter and expressed in joules per kilogram.
The specific heat of vaporization of water is very high: at a temperature of 100°C. For other liquids (alcohol, ether, mercury, kerosene, etc.), the specific heat of vaporization is 3-10 times less.
To convert a liquid mass into vapor requires an amount of heat equal to:
When steam condenses, the same amount of heat is released:
Specific heat of fusion. When a crystalline body melts, all the heat supplied to it goes to increase the potential energy of the molecules. The kinetic energy of the molecules does not change, since melting occurs at a constant temperature.
The amount of heat A required to convert 1 kg of a crystalline substance at the melting point into a liquid of the same temperature is called the specific heat of fusion.
During the crystallization of 1 kg of a substance, exactly the same amount of heat is released. The specific heat of melting of ice is quite high:
In order to melt a crystalline body with a mass, an amount of heat is required equal to:
The amount of heat released during the crystallization of the body is equal to:
1. What is called the amount of heat? 2. What determines the specific heat capacity of substances? 3. What is called the specific heat of vaporization? 4. What is called the specific heat of fusion? 5. In what cases is the amount of transferred heat negative?
