The electric current in the circuit is always manifested by some of its action. This can be both work in a certain load, and the accompanying action of the current. Thus, by the action of the current, one can judge its presence or absence in a given circuit: if the load is working, there is current. If a typical current-related phenomenon is observed, there is current in the circuit, etc.
In general, electric current is capable of causing various actions: thermal, chemical, magnetic (electromagnetic), light or mechanical, and various kinds of current actions often appear simultaneously. These phenomena and actions of the current will be discussed in this article.
Thermal effect of electric current
When a direct or alternating electric current passes through a conductor, the conductor heats up. Such heating conductors under different conditions and applications can be: metals, electrolytes, plasma, metal melts, semiconductors, semimetals.


In the simplest case, if, say, an electric current is passed through a nichrome wire, then it will heat up. This phenomenon is used in heating devices: in electric kettles, boilers, heaters, electric stoves, etc. In electric arc welding, the temperature of the electric arc generally reaches 7000 ° C, and the metal melts easily - this is also the thermal effect of the current.
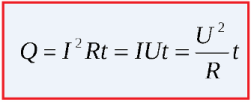

The amount of heat released in the circuit section depends on the voltage applied to this section, the value of the current flowing and on the time of its flow ().
By transforming Ohm's law for a section of the circuit, it is possible to use either voltage or current to calculate the amount of heat, but then it is imperative to know the resistance of the circuit, because it is it that limits the current and causes, in fact, heating. Or, knowing the current and voltage in the circuit, you can just as easily find the amount of heat released.
Chemical action of electric current
Electrolytes containing ions, under the influence of a direct electric current - this is the chemical effect of the current. Negative ions (anions) are attracted to the positive electrode (anode) during electrolysis, and positive ions (cations) are attracted to the negative electrode (cathode). That is, the substances contained in the electrolyte, in the process of electrolysis, are released on the electrodes of the current source.
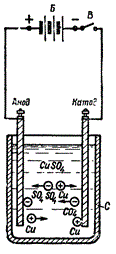
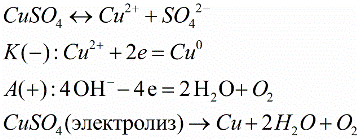
For example, a pair of electrodes is immersed in a solution of a certain acid, alkali or salt, and when an electric current is passed through the circuit, a positive charge is created on one electrode, and a negative charge on the other. The ions contained in the solution begin to be deposited on the electrode with the opposite charge.
For example, during the electrolysis of copper sulfate (CuSO4), copper cations Cu2+ with a positive charge move to a negatively charged cathode, where they receive the missing charge, and become neutral copper atoms, settling on the electrode surface. The hydroxyl group -OH will give up electrons at the anode, and oxygen will be released as a result. Positively charged H+ hydrogen cations and negatively charged SO42- anions will remain in solution.
The chemical action of electric current is used in industry, for example, to decompose water into its constituent parts (hydrogen and oxygen). Also, electrolysis allows you to get some metals in their pure form. With the help of electrolysis, a thin layer of a certain metal (nickel, chromium) is coated on the surface - this, etc.
In 1832, Michael Faraday found that the mass m of the substance released on the electrode is directly proportional to the electric charge q that has passed through the electrolyte. If a direct current I is passed through the electrolyte for a time t, then Faraday's first law of electrolysis is valid:
![]()
Here the coefficient of proportionality k is called the electrochemical equivalent of the substance. It is numerically equal to the mass of the substance released during the passage of a single electric charge through the electrolyte, and depends on the chemical nature of the substance.
In the presence of an electric current in any conductor (solid, liquid or gaseous), a magnetic field is observed around the conductor, that is, a current-carrying conductor acquires magnetic properties.
So, if a magnet is brought to the conductor through which the current flows, for example, in the form of a magnetic compass needle, then the arrow will turn perpendicular to the conductor, and if the conductor is wound on an iron core and a direct current is passed through the conductor, the core will become an electromagnet.
In 1820, Oersted discovered the magnetic effect of current on a magnetic needle, and Ampere established the quantitative laws of the magnetic interaction of conductors with current.
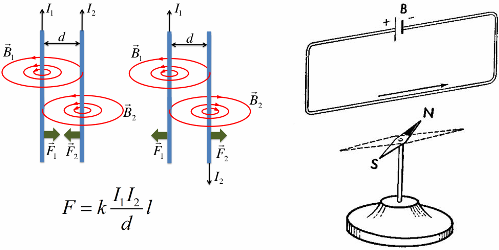
A magnetic field is always generated by current, that is, by moving electric charges, in particular by charged particles (electrons, ions). Oppositely directed currents repel each other, unidirectional currents attract each other.
Such a mechanical interaction occurs due to the interaction of magnetic fields of currents, that is, it is, first of all, a magnetic interaction, and only then a mechanical one. Thus, the magnetic interaction of currents is primary.
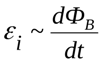
In 1831, Faraday established that a changing magnetic field from one circuit generates a current in another circuit: the generated emf is proportional to the rate of change of the magnetic flux. It is logical that it is the magnetic action of currents that is used to this day in all transformers, and not only in electromagnets (for example, in industrial ones).
In its simplest form, the luminous effect of electric current can be observed in an incandescent lamp, the spiral of which is heated by the current passing through it to white heat and emits light.
For an incandescent lamp, light energy accounts for about 5% of the electricity supplied, the remaining 95% of which is converted into heat.
Fluorescent lamps more efficiently convert current energy into light - up to 20% of electricity is converted into visible light thanks to the phosphor, which receives from an electrical discharge in mercury vapor or in an inert gas such as neon.
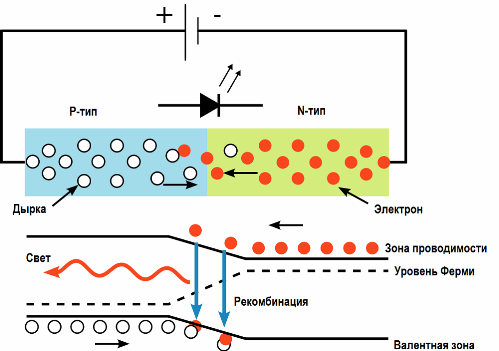
The luminous effect of electric current is realized more effectively in light-emitting diodes. When an electric current is passed through the p-n junction in the forward direction, charge carriers - electrons and holes - recombine with the emission of photons (due to the transition of electrons from one energy level to another).
The best light emitters are direct-gap semiconductors (that is, those that allow direct optical band-to-band transitions), such as GaAs, InP, ZnSe, or CdTe. By varying the composition of semiconductors, it is possible to create LEDs for all possible wavelengths from ultraviolet (GaN) to mid-infrared (PbS). The efficiency of an LED as a light source reaches an average of 50%.
As noted above, each conductor through which an electric current flows forms around itself. Magnetic actions are converted into motion, for example, in electric motors, in magnetic lifting devices, in magnetic valves, in relays, etc.
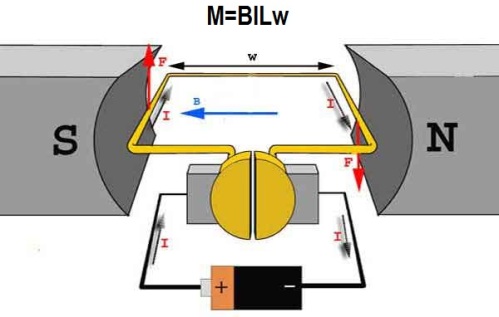
The mechanical action of one current on another describes Ampère's law. This law was first established by André Marie Ampère in 1820 for direct current. From it follows that parallel conductors with electric currents flowing in one direction attract, and in opposite directions they repel.
Ampère's law is also called the law that determines the force with which a magnetic field acts on a small segment of a current-carrying conductor. The force with which the magnetic field acts on a conductor element with current in a magnetic field is directly proportional to the current in the conductor and the vector product of the conductor length element and magnetic induction.
It is based on this principle, where the rotor plays the role of a frame with a current, oriented in the external magnetic field of the stator with a torque M.
