Antoine Laurent LAVOISIER () () Investigated oxygen and created the oxygen theory of combustion, which replaced the phlogiston theory. He studied oxygen and created the oxygen theory of combustion, which replaced the phlogiston theory.

Oxygen is the most common element on Earth In the air 21% (by volume), In the air 21% (by volume), in the earth's crust 49% (by mass), in the earth's crust 49% (by mass), in the hydrosphere 89% ( by mass), in the hydrosphere 89% (by mass), in living organisms up to 65% by mass. in living organisms up to 65% of the mass.

Physical properties Aggregate state - gas under normal conditions. At very low temperatures (-183°C) it turns into a liquid state of aggregation (blue liquid), and at even lower temperatures (-219°C) it becomes solid (blue snow crystals). Aggregate state - gas under normal conditions. At very low temperatures (-183°C) it turns into a liquid state of aggregation (blue liquid), and at even lower temperatures (-219°C) it becomes solid (blue snow crystals). Color - colorless. Color - colorless. Smell - odorless. Smell - odorless. Solubility in water - poorly soluble. Solubility in water - poorly soluble. Heavier than air (M air = 29 g / mol, and M O 2 = 32 g / mol. Heavier than air (M air = 29 g / mol, and M O 2 = 32 g / mol.

Chemical properties Oxygen is a very strong oxidizing agent! It oxidizes many substances already at room temperature (slow oxidation) and even more so when the substance is heated or burned (fast oxidation). Oxygen is a very strong oxidizing agent! It oxidizes many substances already at room temperature (slow oxidation) and even more so when the substance is heated or burned (fast oxidation). In reactions with all elements (except fluorine), oxygen is always an oxidizing agent. In reactions with all elements (except fluorine), oxygen is always an oxidizing agent.

Reactions with metals As a result of the reaction, an oxide of this metal is formed. For example, aluminum is oxidized by oxygen according to the equation: As a result of the reaction, an oxide of this metal is formed. For example, aluminum is oxidized by oxygen according to the equation: t° 4Al + 3O 2 2Al 2 O 3 t° 4Al + 3O 2 2Al 2 O 3 Another example. When lowering a red-hot iron wire into a bottle of oxygen, the wire burns out, spraying to the sides sheaves of sparks - hot particles of iron scale Fe 3 O 4: t ° 3Fe + 2O 2 Fe 3 O 4 t ° 3Fe + 2O 2 Fe 3 O 4


Other examples of reactions with non-metals Combustion of sulfur in oxygen to form sulfur dioxide SO 2: t ° S + O 2 SO 2 t ° S + O 2 SO 2 Combustion of coal in oxygen to form carbon dioxide: Combustion of coal in oxygen to form carbon dioxide: t° C + O 2 CO 2 t° C + O 2 CO 2
Reactions with some complex substances In this case, oxides of the elements that make up the molecule of a complex substance are formed. In this case, oxides of the elements that make up the molecule of a complex substance are formed. For example, when burning copper (II) sulfide For example, when burning copper (II) sulfide t ° 2CuS + 3O 2 2CuO + 2SO 2 t ° 2CuS + 3O 2 2CuO + 2SO 2, two oxides are formed copper (II) oxide and sulfur oxide ( IV). two oxides are formed, copper(II) oxide and sulfur(IV) oxide. During the roasting of sulfides, sulfur oxide is always formed, in which the sulfur valency is IV. During the roasting of sulfides, sulfur oxide is always formed, in which the sulfur valency is IV. Another example is the combustion of methane CH 4. Since this molecule consists of atoms of the elements carbon C and hydrogen H, it means that two oxides are formed carbon monoxide (IV) CO 2 and hydrogen oxide, that is, water - H 2 O: t ° CH 4 + 2O 2 CO 2 + 2H 2 O t ° CH 4 + 2O 2 CO 2 + 2H 2 O

The chemical interaction of a substance with oxygen is called an oxidation reaction. Oxidation reactions accompanied by the release of heat and light are called combustion reactions. Combustion reactions of substances are examples of rapid oxidation, but rotting, rusting, etc. these are examples of slow oxidation of substances with oxygen. Combustion reactions of substances are examples of fast oxidation, but rotting, rusting, etc. these are examples of the slow oxidation of substances by oxygen

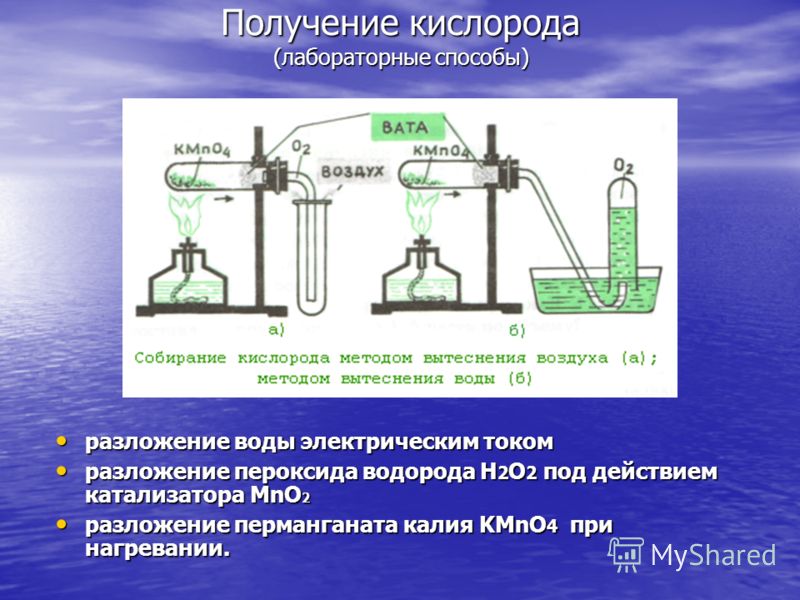
Oxygen production (laboratory methods) water decomposition by electric current water decomposition by electric current decomposition of hydrogen peroxide H 2 O 2 under the action of MnO 2 catalyst decomposition of hydrogen peroxide H 2 O 2 under the action of MnO 2 catalyst decomposition of potassium permanganate KMnO 4 when heated. decomposition of potassium permanganate KMnO 4 when heated.
Obtaining oxygen (industrial method) In industry, to obtain pure oxygen, liquid air distillation is used, based on different boiling temperatures of the air components. The air is cooled to about -200°C and then slowly heated. When the temperature reaches -183°C, oxygen escapes from the liquid air, the remaining components of the liquefied air at this temperature remain in a liquid state of aggregation. In industry, to obtain pure oxygen, liquid air distillation is used, based on different boiling temperatures of the air components. The air is cooled to about -200°C and then slowly heated. When the temperature reaches -183°C, oxygen escapes from the liquid air, the remaining components of the liquefied air at this temperature remain in a liquid state of aggregation.

The use of oxygen in construction and mechanical engineering in construction and mechanical engineering - for oxy-acetylene gas welding and gas cutting of metals - for oxy-acetylene gas welding and gas cutting of metals - for spraying and surfacing metals in oil production in oil production - when injected into the formation to increase the displacement energy in metallurgy and mining industry in metallurgy and mining industry - in convective steel production, oxygen blast in blast furnaces, extraction of gold and ores, production of ferroalloys, smelting of nickel, zinc, lead, zirconium and other non-ferrous metals - in convective steel production, oxygen blast in blast furnaces , extraction of gold and ores, production of ferroalloys, smelting of nickel, zinc, lead, zirconium and other non-ferrous metals - with direct reduction of iron - with direct reduction of iron - with fire cleaning in foundry production - with fire cleaning in foundry production - with fire drilling x breeds

The use of oxygen in medicine in medicine - in oxybaric chambers - in oxybaric chambers - when filling oxygen masks, pillows, etc. - when filling oxygen masks, pillows, etc. - in wards with a special microclimate - in wards with a special microclimate - for the production of oxygen cocktails - for the production of oxygen cocktails - in the cultivation of microorganisms - in the cultivation of microorganisms in ecology in ecology - in the purification of drinking water - in the purification of drinking water - in the recycling of metals - when recycling metals - when blowing wastewater with oxygen - when blowing wastewater with oxygen - when neutralizing chemically active waste in treatment plants in incinerators - when neutralizing chemically active waste in treatment plants in incinerators

The use of oxygen in the chemical industry in the chemical industry - in the production of acetylene, cellulose, methyl alcohol, ammonia, nitric and sulfuric acid - in the production of acetylene, cellulose, methyl alcohol, ammonia, nitric and sulfuric acid - in the catalytic conversion of natural gas (in the production of synthetic ammonia) - in the catalytic conversion of natural gas (in the production of synthetic ammonia) - in the high-temperature conversion of methane - in the high-temperature conversion of methane in the power industry in the power industry - in the gasification of solid fuels - in the gasification of solid fuels - for air enrichment for domestic and industrial boilers - for enrichment air for domestic and industrial boilers - for compressing water-coal mixture - for compressing water-coal mixture

The use of oxygen in military equipment in military equipment - in pressure chambers - in pressure chambers - for the operation of diesel engines under water - for the operation of diesel engines under water - as a fuel oxidizer for rocket engines - as a fuel oxidizer for rocket engines in agriculture in agriculture - for enriching the aquatic environment with oxygen in fishing - for enriching the aquatic environment with oxygen in fishing - in the manufacture of oxygen cocktails - in the manufacture of oxygen cocktails - for animal weight gain - for animal weight gain

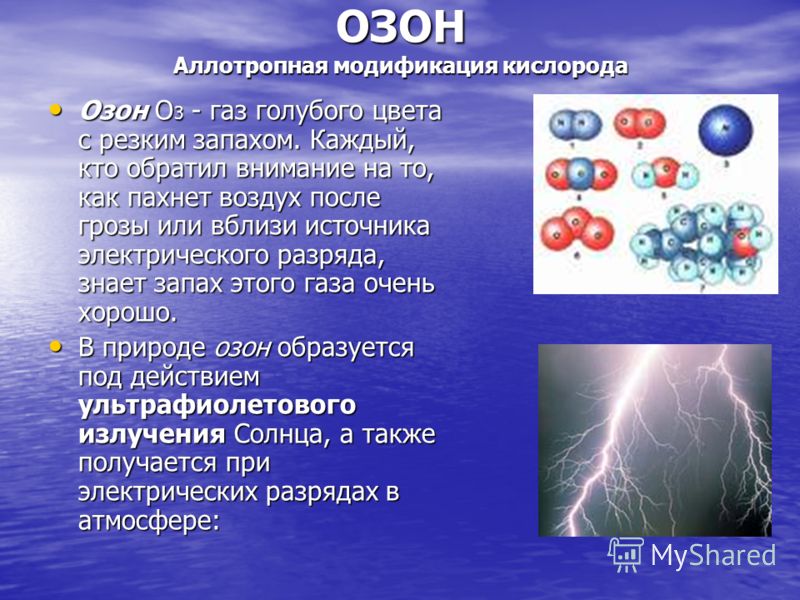
OZONE Allotropic modification of oxygen Ozone O 3 is a blue gas with a pungent odor. Anyone who has paid attention to how the air smells after a thunderstorm or near a source of electrical discharge knows the smell of this gas very well. Ozone O 3 is a blue gas with a pungent odor. Anyone who has paid attention to how the air smells after a thunderstorm or near a source of electrical discharge knows the smell of this gas very well. In nature, ozone is formed by the action of ultraviolet radiation from the Sun, and is also produced by electrical discharges in the atmosphere:
Ozone is a very strong oxidizing agent, so it is used in the disinfection of drinking water. On contact with most oxidizable substances, an explosion occurs. Ozone is formed in the Earth's atmosphere at a height of 25 km under the influence of solar radiation, it absorbs dangerous radiation from the Sun. However, in the ozone "umbrella" of the Earth, only about 30 meters thick, "holes" appear every now and then. More and more gases "harmful" to ozone, such as nitrogen monoxide NO or those substances that are used to fill refrigeration units and aerosol cans, are getting into the air. Even the partial disappearance of the ozone layer above the Earth threatens all living things with death ... However, in the ozone "umbrella" of the Earth, only about 30 meters thick, "holes" appear every now and then. More and more gases "harmful" to ozone, such as nitrogen monoxide NO or those substances that are used to fill refrigeration units and aerosol cans, are getting into the air. Even the partial disappearance of the ozone layer above the Earth threatens all living things with death...


