Nucleons in nuclei are in states that differ significantly from their free states. With the exception of the ordinary hydrogen nucleus, in all cores there are at least two nucleons between which there is a special nuclear strong force – attraction, which ensures the stability of nuclei despite the repulsion of like-charged protons.
· The binding energy of the nucleon in the nucleus is called a physical quantity equal to the work that must be done to remove the nucleon from the nucleus without imparting kinetic energy to it.
· Core binding energy determined by the amount of that work,to be done,to split the nucleus into its constituent nucleons without imparting kinetic energy to them.
It follows from the law of conservation of energy that during the formation of a nucleus, such energy must be released that must be spent when the nucleus splits into its constituent nucleons. The nuclear binding energy is the difference between the energy of all free nucleons that make up the nucleus and their energy in the nucleus.
When a nucleus is formed, its mass decreases: the mass of the nucleus is less than the sum of the masses of its constituent nucleons. The decrease in the mass of the nucleus during its formation is explained by the release of binding energy. If a W sv is the amount of energy released during the formation of the nucleus, then the corresponding mass
| (9.2.1) |
called mass defect and characterizes the decrease in the total mass during the formation of a nucleus from its constituent nucleons.
If the nucleus has a mass M poison formed from Z protons with mass m p and from ( A – Z) neutrons with mass m n, then:
| . | (9.2.2) |
Instead of the mass of the nucleus M poison value ∆ m can be expressed in terms of atomic mass M at:
| , | (9.2.3) |
where mH is the mass of the hydrogen atom. In practical calculation, ∆ m the masses of all particles and atoms are expressed in terms of atomic mass units (a.u.m.). One atomic mass unit corresponds to an atomic energy unit (a.e.e.): 1 a.u.e. = 931.5016 MeV.
The mass defect serves as a measure of the nuclear binding energy:
| . | (9.2.4) |
The specific binding energy of the nucleus ω St is called binding energy,per nucleon:
| . | (9.2.5) |
The value of ω St is 8 MeV/nucleon on average. On fig. 9.2 shows the dependence of the specific binding energy on the mass number A, which characterizes the different bond strengths of nucleons in the nuclei of different chemical elements. The nuclei of elements in the middle part of the periodic system (), i.e. from to , the most durable.
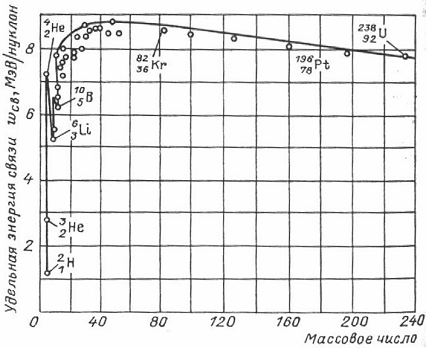
In these nuclei, ω is close to 8.7 MeV/nucleon. As the number of nucleons in the nucleus increases, the specific binding energy decreases. The nuclei of atoms of chemical elements located at the end of the periodic system (for example, the nucleus of uranium) have ω St ≈ 7.6 MeV / nucleon. This explains the possibility of energy release during the fission of heavy nuclei. In the region of small mass numbers, there are sharp "peaks" of the specific binding energy. The maxima are typical for nuclei with even numbers of protons and neutrons ( , , ), the minima are for nuclei with odd numbers of protons and neutrons ( , , ).
If the nucleus has the lowest possible energy, then it is located in basic energy state . If the nucleus has energy , then it is located in excited energy state . The case corresponds to the splitting of the nucleus into its constituent nucleons. Unlike the energy levels of an atom, which are separated by units of electron volts, the energy levels of the nucleus are separated from each other by a mega-electron volt (MeV). This explains the origin and properties of gamma radiation.
Data on the binding energy of nuclei and the use of a drop model of the nucleus made it possible to establish some regularities in the structure of atomic nuclei.
The criterion for the stability of atomic nuclei is the ratio between the number of protons and neutrons in a stable core for isobars data (). The condition for minimum nuclear energy leads to the following relation between Z mouth and BUT:
|
|
(9.2.6) |
Take an integer Z mouth closest to the one obtained by this formula.
For small and medium values BUT the numbers of neutrons and protons in stable nuclei are approximately the same: Z ≈ BUT – Z.
With growth Z the Coulomb repulsive forces of protons grow proportionally Z·( Z – 1) ~ Z 2 (pair interaction of protons), and to compensate for this repulsion by nuclear attraction, the number of neutrons must increase faster than the number of protons.
To view demos, click on the appropriate hyperlink:
