Man on skis, and without them.
On loose snow, a person walks with great difficulty, sinking deeply at every step. But, having put on skis, he can walk, almost without falling into it. Why? On skis or without skis, a person acts on the snow with the same force equal to his own weight. However, the effect of this force in both cases is different, because the surface area on which the person presses is different, with and without skis. The surface area of the ski is almost 20 times the area of the sole. Therefore, standing on skis, a person acts on each square centimeter of the snow surface area with a force 20 times less than standing on the snow without skis.
The student, pinning a newspaper to the board with buttons, acts on each button with the same force. However, a button with a sharper end is easier to enter into the tree.
This means that the result of the action of a force depends not only on its modulus, direction and point of application, but also on the area of the surface to which it is applied (perpendicular to which it acts).
This conclusion is confirmed by physical experiments.

Experience. The result of this force depends on what force acts per unit area of the surface.
Nails must be driven into the corners of a small board. First, we set the nails driven into the board on the sand with their points up and put a weight on the board. In this case, the nail heads are only slightly pressed into the sand. Then turn the board over and put the nails on the tip. In this case, the area of support is smaller, and under the action of the same force, the nails go deep into the sand.

An experience. Second illustration.
The result of the action of this force depends on what force acts on each unit of surface area.
In the considered examples, the forces acted perpendicular to the surface of the body. The person's weight was perpendicular to the surface of the snow; the force acting on the button is perpendicular to the surface of the board.
The value equal to the ratio of the force acting perpendicular to the surface to the area of \u200b\u200bthis surface is called pressure.
To determine the pressure, it is necessary to divide the force acting perpendicular to the surface by the surface area:
pressure = force / area.
Let us denote the quantities included in this expression: pressure - p, the force acting on the surface, - F and the surface area S.
Then we get the formula:
p = F/S
It is clear that a larger force acting on the same area will produce more pressure.
The pressure unit is taken as the pressure that produces a force of 1 N acting on a surface of 1 m 2 perpendicular to this surface.
Unit of pressure - newton per square meter(1 N / m 2). In honor of the French scientist Blaise Pascal it's called pascal Pa). In this way,
1 Pa = 1 N / m 2.
Other pressure units are also used: hectopascal (hPa) and kilopascal (kPa).
1 kPa = 1000 Pa;
1 hPa = 100 Pa;
1 Pa = 0.001 kPa;
1 Pa = 0.01 hPa.
Let's write down the condition of the problem and solve it.
Given : m = 45 kg, S = 300 cm 2; p = ?
In SI units: S = 0.03 m 2
Solution:
p = F/S,
F = P,
P = g m,
P= 9.8 N 45 kg ≈ 450 N,
p\u003d 450 / 0.03 N / m 2 \u003d 15000 Pa \u003d 15 kPa
"Answer": p = 15000 Pa = 15 kPa
Ways to reduce and increase pressure.
A heavy caterpillar tractor produces a pressure on the soil equal to 40-50 kPa, that is, only 2-3 times more than the pressure of a boy weighing 45 kg. This is because the weight of the tractor is distributed over a larger area due to the caterpillar drive. And we have established that the larger the area of the support, the less pressure produced by the same force on this support .
Depending on whether you need to get a small or a large pressure, the area of \u200b\u200bsupport increases or decreases. For example, in order for the soil to withstand the pressure of a building being erected, the area of \u200b\u200bthe lower part of the foundation is increased.
Truck tires and aircraft chassis are made much wider than passenger cars. Particularly wide tires are made for cars designed to travel in deserts.
Heavy machines, like a tractor, a tank or a swamp, having a large bearing area of the tracks, pass through swampy terrain that a person cannot pass through.
On the other hand, with a small surface area, a large pressure can be generated with a small force. For example, pressing a button into a board, we act on it with a force of about 50 N. Since the area of the button tip is approximately 1 mm 2, the pressure produced by it is equal to:
p \u003d 50 N / 0.000001 m 2 \u003d 50,000,000 Pa \u003d 50,000 kPa.
For comparison, this pressure is 1000 times more than the pressure exerted by a caterpillar tractor on the soil. Many more such examples can be found.
The blade of cutting and piercing tools (knives, scissors, cutters, saws, needles, etc.) is specially sharpened. The sharpened edge of a sharp blade has a small area, so even a small force creates a lot of pressure, and it is easy to work with such a tool.
Cutting and piercing devices are also found in wildlife: these are teeth, claws, beaks, spikes, etc. - they are all made of hard material, smooth and very sharp.
Pressure

It is known that gas molecules move randomly.
We already know that gases, unlike solids and liquids, fill the entire vessel in which they are located. For example, a steel cylinder for storing gases, a car tire tube or a volleyball. In this case, the gas exerts pressure on the walls, bottom and lid of the cylinder, chamber or any other body in which it is located. Gas pressure is due to other reasons than the pressure of a solid body on a support.
It is known that gas molecules move randomly. During their movement, they collide with each other, as well as with the walls of the vessel in which the gas is located. There are many molecules in the gas, and therefore the number of their impacts is very large. For example, the number of hits of air molecules in a room on a surface of 1 cm 2 in 1 s is expressed as a twenty-three-digit number. Although the impact force of an individual molecule is small, the action of all molecules on the walls of the vessel is significant - it creates gas pressure.
So, gas pressure on the walls of the vessel (and on the body placed in the gas) is caused by impacts of gas molecules .
Consider the following experience. Place a rubber ball under the air pump bell. It contains a small amount of air and has an irregular shape. Then we pump out the air from under the bell with a pump. The shell of the ball, around which the air becomes more and more rarefied, gradually swells and takes the form of a regular ball.
How to explain this experience?

Special durable steel cylinders are used for storage and transportation of compressed gas.
In our experiment, moving gas molecules continuously hit the walls of the ball inside and out. When air is pumped out, the number of molecules in the bell around the shell of the ball decreases. But inside the ball their number does not change. Therefore, the number of impacts of molecules on the outer walls of the shell becomes less than the number of impacts on the inner walls. The balloon is inflated until the force of elasticity of its rubber shell becomes equal to the pressure force of the gas. The shell of the ball takes the shape of a ball. This shows that gas presses on its walls equally in all directions. In other words, the number of molecular impacts per square centimeter of surface area is the same in all directions. The same pressure in all directions is characteristic of a gas and is a consequence of the random movement of a huge number of molecules.
Let's try to reduce the volume of gas, but so that its mass remains unchanged. This means that in each cubic centimeter of gas there will be more molecules, the density of the gas will increase. Then the number of impacts of molecules on the walls will increase, i.e., the gas pressure will increase. This can be confirmed by experience.
On the image a A glass tube is shown, one end of which is covered with a thin rubber film. A piston is inserted into the tube. When the piston is pushed in, the volume of air in the tube decreases, i.e., the gas is compressed. The rubber film bulges outward, indicating that the air pressure in the tube has increased.
On the contrary, with an increase in the volume of the same mass of gas, the number of molecules in each cubic centimeter decreases. This will reduce the number of impacts on the walls of the vessel - the pressure of the gas will become less. Indeed, when the piston is pulled out of the tube, the volume of air increases, the film bends inside the vessel. This indicates a decrease in air pressure in the tube. The same phenomena would be observed if instead of air in the tube there would be any other gas.
So, when the volume of a gas decreases, its pressure increases, and when the volume increases, the pressure decreases, provided that the mass and temperature of the gas remain unchanged.
How does the pressure of a gas change when it is heated at a constant volume? It is known that the speed of movement of gas molecules increases when heated. Moving faster, the molecules will hit the walls of the vessel more often. In addition, each impact of the molecule on the wall will be stronger. As a result, the walls of the vessel will experience more pressure.
Consequently, The pressure of a gas in a closed vessel is greater the higher the temperature of the gas, provided that the mass of the gas and the volume do not change.
From these experiments it can be concluded that the pressure of the gas is greater, the more often and stronger the molecules hit the walls of the vessel .
For storage and transportation of gases, they are highly compressed. At the same time, their pressure increases, gases must be enclosed in special, very durable cylinders. Such cylinders, for example, contain compressed air in submarines, oxygen used in metal welding. Of course, we must always remember that gas cylinders cannot be heated, especially when they are filled with gas. Because, as we already understand, an explosion can occur with very unpleasant consequences.
Pascal's law.

Pressure is transmitted to each point of the liquid or gas.

The pressure of the piston is transmitted to each point of the liquid filling the ball.

Now gas.
Unlike solids, individual layers and small particles of liquid and gas can move freely relative to each other in all directions. It is enough, for example, to lightly blow on the surface of the water in a glass to cause the water to move. Ripples appear on a river or lake at the slightest breeze.
The mobility of gas and liquid particles explains that the pressure produced on them is transmitted not only in the direction of the force, but at every point. Let's consider this phenomenon in more detail.
On the image, a a vessel containing a gas (or liquid) is depicted. The particles are evenly distributed throughout the vessel. The vessel is closed by a piston that can move up and down.
By applying some force, let's make the piston move a little inward and compress the gas (liquid) directly below it. Then the particles (molecules) will be located in this place more densely than before (Fig., b). Due to the mobility of the gas particles will move in all directions. As a result, their arrangement will again become uniform, but more dense than before (Fig. c). Therefore, the pressure of the gas will increase everywhere. This means that additional pressure is transferred to all particles of a gas or liquid. So, if the pressure on the gas (liquid) near the piston itself increases by 1 Pa, then at all points inside gas or liquid pressure will be greater than before by the same amount. The pressure on the walls of the vessel, and on the bottom, and on the piston will increase by 1 Pa.
The pressure exerted on a liquid or gas is transmitted to any point equally in all directions .
This statement is called Pascal's law.
Based on Pascal's law, it is easy to explain the following experiments.
The figure shows a hollow sphere with small holes in various places. A tube is attached to the ball, into which a piston is inserted. If you draw water into the ball and push the piston into the tube, then water will flow from all the holes in the ball. In this experiment, the piston presses on the surface of the water in the tube. The water particles under the piston, condensing, transfer its pressure to other layers lying deeper. Thus, the pressure of the piston is transmitted to each point of the liquid filling the ball. As a result, part of the water is pushed out of the ball in the form of identical streams flowing from all holes.
If the ball is filled with smoke, then when the piston is pushed into the tube, identical streams of smoke will begin to come out of all the holes in the ball. This confirms that and gases transmit the pressure produced on them equally in all directions.
Pressure in liquid and gas.

Under the weight of the liquid, the rubber bottom in the tube will sag.
Liquids, like all bodies on Earth, are affected by the force of gravity. Therefore, each layer of liquid poured into a vessel creates pressure with its weight, which, according to Pascal's law, is transmitted in all directions. Therefore, there is pressure inside the liquid. This can be verified by experience.
Pour water into a glass tube, the bottom hole of which is closed with a thin rubber film. Under the weight of the liquid, the bottom of the tube will bend.
Experience shows that the higher the column of water above the rubber film, the more it sags. But every time after the rubber bottom sags, the water in the tube comes to equilibrium (stops), because, in addition to gravity, the elastic force of the stretched rubber film acts on the water.
Forces acting on the rubber film |
are the same on both sides. |

Illustration.
The bottom moves away from the cylinder due to the pressure on it due to gravity.
Let's lower a tube with a rubber bottom, into which water is poured, into another, wider vessel with water. We will see that as the tube is lowered, the rubber film gradually straightens out. Full straightening of the film shows that the forces acting on it from above and below are equal. Full straightening of the film occurs when the water levels in the tube and vessel coincide.
The same experiment can be carried out with a tube in which a rubber film closes the side opening, as shown in figure a. Immerse this tube of water into another vessel of water, as shown in the figure, b. We will notice that the film straightens again as soon as the water levels in the tube and vessel are equal. This means that the forces acting on the rubber film are the same from all sides.
Take a vessel whose bottom can fall off. Let's put it in a jar of water. In this case, the bottom will be tightly pressed to the edge of the vessel and will not fall off. It is pressed by the force of water pressure, directed from the bottom up.
We will carefully pour water into the vessel and watch its bottom. As soon as the level of water in the vessel coincides with the level of water in the jar, it will fall away from the vessel.
At the moment of separation, a column of liquid in the vessel presses down on the bottom, and pressure is transmitted from bottom to top to the bottom of the same column of liquid in height, but located in the jar. Both these pressures are the same, but the bottom moves away from the cylinder due to the action of its own gravity on it.
The experiments with water were described above, but if we take any other liquid instead of water, the results of the experiment will be the same.
So, experiments show that inside the liquid there is pressure, and at the same level it is the same in all directions. Pressure increases with depth.
Gases do not differ in this respect from liquids, because they also have weight. But we must remember that the density of a gas is hundreds of times less than the density of a liquid. The weight of the gas in the vessel is small, and in many cases its "weight" pressure can be ignored.
Calculation of liquid pressure on the bottom and walls of the vessel.

Calculation of liquid pressure on the bottom and walls of the vessel.
Consider how you can calculate the pressure of a liquid on the bottom and walls of a vessel. Let us first solve the problem for a vessel having the shape of a rectangular parallelepiped.
Strength F, with which the liquid poured into this vessel presses on its bottom, is equal to the weight P the liquid in the vessel. The weight of a liquid can be determined by knowing its mass. m. Mass, as you know, can be calculated by the formula: m = ρ V. The volume of liquid poured into the vessel we have chosen is easy to calculate. If the height of the liquid column in the vessel is denoted by the letter h, and the area of the bottom of the vessel S, then V = S h.
Liquid mass m = ρ V, or m = ρ S h .
The weight of this fluid P = g m, or P = g ρ S h.
Since the weight of the liquid column is equal to the force with which the liquid presses on the bottom of the vessel, then, dividing the weight P To the square S, we get the fluid pressure p:
p = P/S , or p = g ρ S h/S,
We have obtained a formula for calculating the pressure of a liquid on the bottom of a vessel. From this formula it can be seen that the pressure of a liquid at the bottom of a vessel depends only on the density and height of the liquid column.
Therefore, according to the derived formula, it is possible to calculate the pressure of a liquid poured into a vessel any form(Strictly speaking, our calculation is only suitable for vessels having the shape of a straight prism and a cylinder. In physics courses for the institute, it was proved that the formula is also true for a vessel of arbitrary shape). In addition, it can be used to calculate the pressure on the walls of the vessel. The pressure inside the fluid, including pressure from bottom to top, is also calculated using this formula, since the pressure at the same depth is the same in all directions.
When calculating pressure using the formula p = gph need density ρ expressed in kilograms per cubic meter (kg / m 3), and the height of the liquid column h- in meters (m), g\u003d 9.8 N / kg, then the pressure will be expressed in pascals (Pa).
Example. Determine the oil pressure at the bottom of the tank if the height of the oil column is 10 m and its density is 800 kg/m 3 .
Let's write down the condition of the problem and write it down.
Given :
ρ \u003d 800 kg / m 3
Solution :
p = 9.8 N/kg 800 kg/m 3 10 m ≈ 80,000 Pa ≈ 80 kPa.
Answer : p ≈ 80 kPa.
Communicating vessels.

Communicating vessels.
The figure shows two vessels connected to each other by a rubber tube. Such vessels are called communicating. A watering can, a teapot, a coffee pot are examples of communicating vessels. We know from experience that water poured, for example, into a watering can, always stands at the same level in the spout and inside.
Communicating vessels are common to us. For example, it can be a teapot, a watering can or a coffee pot. |
The surfaces of a homogeneous liquid are installed at the same level in communicating vessels of any shape. |
Liquids of various densities. |
With communicating vessels, the following simple experiment can be done. At the beginning of the experiment, we clamp the rubber tube in the middle, and pour water into one of the tubes. Then we open the clamp, and the water instantly flows into the other tube until the water surfaces in both tubes are at the same level. You can fix one of the tubes in a tripod, and raise, lower or tilt the other in different directions. And in this case, as soon as the liquid calms down, its levels in both tubes will equalize.
In communicating vessels of any shape and section, the surfaces of a homogeneous liquid are set at the same level(provided that the air pressure over the liquid is the same) (Fig. 109).
This can be justified as follows. The liquid is at rest without moving from one vessel to another. This means that the pressures in both vessels are the same at any level. The liquid in both vessels is the same, that is, it has the same density. Therefore, its heights must also be the same. When we raise one vessel or add liquid to it, the pressure in it increases and the liquid moves into another vessel until the pressures are balanced.
If a liquid of one density is poured into one of the communicating vessels, and another density is poured into the second, then at equilibrium the levels of these liquids will not be the same. And this is understandable. We know that the pressure of a liquid on the bottom of a vessel is directly proportional to the height of the column and the density of the liquid. And in this case, the densities of the liquids will be different.
With equal pressures, the height of a liquid column with a higher density will be less than the height of a liquid column with a lower density (Fig.).

An experience. How to determine the mass of air.
Air weight. Atmosphere pressure.

existence of atmospheric pressure.

Atmospheric pressure is greater than the pressure of rarefied air in a vessel.
The force of gravity acts on the air, as well as on any body located on the Earth, and, therefore, the air has weight. The weight of air is easy to calculate, knowing its mass.
We will show by experience how to calculate the mass of air. To do this, take a strong glass ball with a cork and a rubber tube with a clamp. We pump air out of it with a pump, clamp the tube with a clamp and balance it on the scales. Then, opening the clamp on the rubber tube, let air into it. In this case, the balance of the scales will be disturbed. To restore it, weights will have to be placed on another scale pan, the mass of which will be equal to the mass of air in the volume of the ball.
Experiments have established that at a temperature of 0 ° C and normal atmospheric pressure, the mass of air with a volume of 1 m 3 is 1.29 kg. The weight of this air is easy to calculate:
P = g m, P = 9.8 N/kg 1.29 kg ≈ 13 N.
The air envelope that surrounds the earth is called atmosphere (from Greek. atmosphere steam, air, and sphere- ball).
The atmosphere, as shown by observations of the flight of artificial satellites of the Earth, extends to a height of several thousand kilometers.
Due to the action of gravity, the upper layers of the atmosphere, like ocean water, compress the lower layers. The air layer adjacent directly to the Earth is compressed the most and, according to Pascal's law, transfers the pressure produced on it in all directions.
As a result of this, the earth's surface and the bodies on it experience the pressure of the entire thickness of the air, or, as is usually said in such cases, experience Atmosphere pressure .
The existence of atmospheric pressure can be explained by many phenomena that we encounter in life. Let's consider some of them.
The figure shows a glass tube, inside of which there is a piston that fits snugly against the walls of the tube. The end of the tube is dipped in water. If you raise the piston, then the water will rise behind it.
This phenomenon is used in water pumps and some other devices.
The figure shows a cylindrical vessel. It is closed with a cork into which a tube with a tap is inserted. Air is pumped out of the vessel by a pump. The end of the tube is then placed in water. If you now open the tap, then the water will splash into the inside of the vessel in a fountain. Water enters the vessel because the atmospheric pressure is greater than the pressure of rarefied air in the vessel.
Why does the air shell of the Earth exist.
Like all bodies, the molecules of gases that make up the air envelope of the Earth are attracted to the Earth.
But why, then, do they not all fall to the surface of the Earth? How is the air shell of the Earth, its atmosphere, preserved? To understand this, we must take into account that the molecules of gases are in continuous and random motion. But then another question arises: why these molecules do not fly away into the world space, that is, into space.
In order to completely leave the Earth, a molecule, like a spacecraft or a rocket, must have a very high speed (not less than 11.2 km/s). This so-called second escape velocity. The speed of most molecules in the Earth's air envelope is much less than this cosmic speed. Therefore, most of them are tied to the Earth by gravity, only a negligible number of molecules fly beyond the Earth into space.
The random movement of molecules and the effect of gravity on them result in the fact that gas molecules "float" in space near the Earth, forming an air shell, or the atmosphere known to us.
Measurements show that air density decreases rapidly with height. So, at a height of 5.5 km above the Earth, the air density is 2 times less than its density at the Earth's surface, at a height of 11 km - 4 times less, etc. The higher, the rarer the air. And finally, in the uppermost layers (hundreds and thousands of kilometers above the Earth), the atmosphere gradually turns into airless space. The air shell of the Earth does not have a clear boundary.
Strictly speaking, due to the action of gravity, the density of the gas in any closed vessel is not the same throughout the entire volume of the vessel. At the bottom of the vessel, the density of the gas is greater than in its upper parts, and therefore the pressure in the vessel is not the same. It is larger at the bottom of the vessel than at the top. However, for the gas contained in the vessel, this difference in density and pressure is so small that in many cases it can be completely ignored, just be aware of it. But for an atmosphere extending over several thousand kilometers, the difference is significant.
Measurement of atmospheric pressure. The Torricelli experience.
It is impossible to calculate atmospheric pressure using the formula for calculating the pressure of a liquid column (§ 38). For such a calculation, you need to know the height of the atmosphere and the density of the air. But the atmosphere does not have a definite boundary, and the air density at different heights is different. However, atmospheric pressure can be measured using an experiment proposed in the 17th century by an Italian scientist. Evangelista Torricelli a student of Galileo.
Torricelli's experiment is as follows: a glass tube about 1 m long, sealed at one end, is filled with mercury. Then, tightly closing the second end of the tube, it is turned over and lowered into a cup with mercury, where this end of the tube is opened under the level of mercury. As in any liquid experiment, part of the mercury is poured into the cup, and part of it remains in the tube. The height of the mercury column remaining in the tube is approximately 760 mm. There is no air above the mercury inside the tube, there is an airless space, so no gas exerts pressure from above on the mercury column inside this tube and does not affect the measurements.
Torricelli, who proposed the experience described above, also gave his explanation. The atmosphere presses on the surface of the mercury in the cup. Mercury is in balance. This means that the pressure in the tube is aa 1 (see figure) is equal to atmospheric pressure. When atmospheric pressure changes, the height of the mercury column in the tube also changes. As the pressure increases, the column lengthens. As the pressure decreases, the mercury column decreases in height.
The pressure in the tube at the level aa1 is created by the weight of the mercury column in the tube, since there is no air above the mercury in the upper part of the tube. Hence it follows that atmospheric pressure is equal to the pressure of the mercury column in the tube , i.e.
p atm = p mercury.
The greater the atmospheric pressure, the higher the mercury column in Torricelli's experiment. Therefore, in practice, atmospheric pressure can be measured by the height of the mercury column (in millimeters or centimeters). If, for example, atmospheric pressure is 780 mm Hg. Art. (they say "millimeters of mercury"), this means that the air produces the same pressure as a vertical column of mercury 780 mm high produces.
Therefore, in this case, 1 millimeter of mercury (1 mm Hg) is taken as the unit of atmospheric pressure. Let's find the relationship between this unit and the unit known to us - pascal(Pa).
The pressure of a mercury column ρ of mercury with a height of 1 mm is:
p = g ρ h, p\u003d 9.8 N / kg 13,600 kg / m 3 0.001 m ≈ 133.3 Pa.
So, 1 mm Hg. Art. = 133.3 Pa.
Currently, atmospheric pressure is usually measured in hectopascals (1 hPa = 100 Pa). For example, weather reports may announce that the pressure is 1013 hPa, which is the same as 760 mmHg. Art.
Observing daily the height of the mercury column in the tube, Torricelli discovered that this height changes, that is, atmospheric pressure is not constant, it can increase and decrease. Torricelli also noticed that atmospheric pressure is related to changes in the weather.
If you attach a vertical scale to the mercury tube used in Torricelli's experiment, you get the simplest device - mercury barometer (from Greek. baros- heaviness, metreo- measure). It is used to measure atmospheric pressure.
Barometer - aneroid.


In practice, a metal barometer is used to measure atmospheric pressure, called aneroid (translated from Greek - aneroid). The barometer is called so because it does not contain mercury.
The appearance of the aneroid is shown in the figure. Its main part is a metal box 1 with a wavy (corrugated) surface (see other fig.). Air is pumped out of this box, and so that atmospheric pressure does not crush the box, its cover 2 is pulled up by a spring. As atmospheric pressure increases, the lid flexes downward and tensions the spring. When the pressure decreases, the spring straightens the cover. An arrow-pointer 4 is attached to the spring by means of a transmission mechanism 3, which moves to the right or left when the pressure changes. A scale is fixed under the arrow, the divisions of which are marked according to the indications of a mercury barometer. So, the number 750, against which the aneroid needle stands (see Fig.), shows that at the given moment in the mercury barometer the height of the mercury column is 750 mm.
Therefore, atmospheric pressure is 750 mm Hg. Art. or ≈ 1000 hPa.
The value of atmospheric pressure is very important for predicting the weather for the coming days, since changes in atmospheric pressure are associated with changes in the weather. A barometer is a necessary instrument for meteorological observations.
Atmospheric pressure at various altitudes.
In a liquid, the pressure, as we know, depends on the density of the liquid and the height of its column. Due to the low compressibility, the density of the liquid at different depths is almost the same. Therefore, when calculating the pressure, we consider its density to be constant and take into account only the change in height.
The situation is more complicated with gases. Gases are highly compressible. And the more the gas is compressed, the greater its density, and the greater the pressure it produces. After all, the pressure of a gas is created by the impact of its molecules on the surface of the body.
The layers of air near the surface of the Earth are compressed by all the overlying layers of air above them. But the higher the layer of air from the surface, the weaker it is compressed, the lower its density. Hence, the less pressure it produces. If, for example, a balloon rises above the surface of the Earth, then the air pressure on the balloon becomes less. This happens not only because the height of the air column above it decreases, but also because the air density decreases. It is smaller at the top than at the bottom. Therefore, the dependence of air pressure on altitude is more complicated than that of liquids.
Observations show that atmospheric pressure in areas lying at sea level is on average 760 mm Hg. Art.
Atmospheric pressure equal to the pressure of a mercury column 760 mm high at a temperature of 0 ° C is called normal atmospheric pressure..
normal atmospheric pressure equals 101 300 Pa = 1013 hPa.
The higher the altitude, the lower the pressure.
With small rises, on average, for every 12 m of rise, the pressure decreases by 1 mm Hg. Art. (or 1.33 hPa).
Knowing the dependence of pressure on altitude, it is possible to determine the height above sea level by changing the readings of the barometer. Aneroids having a scale on which you can directly measure the height above sea level are called altimeters . They are used in aviation and when climbing mountains.
Pressure gauges.

We already know that barometers are used to measure atmospheric pressure. To measure pressures greater or less than atmospheric pressure, the pressure gauges (from Greek. manos- rare, inconspicuous metreo- measure). Pressure gauges are liquid and metal.
|
|
Consider first the device and action open liquid manometer. It consists of a two-legged glass tube into which some liquid is poured. The liquid is installed in both knees at the same level, since only atmospheric pressure acts on its surface in the knees of the vessel.


To understand how such a pressure gauge works, it can be connected with a rubber tube to a round flat box, one side of which is covered with a rubber film. If you press your finger on the film, then the liquid level in the manometer knee connected in the box will decrease, and in the other knee it will increase. What explains this?
Pressing on the film increases the air pressure in the box. According to Pascal's law, this increase in pressure is also transmitted to the liquid in that knee of the pressure gauge, which is attached to the box. Therefore, the pressure on the liquid in this knee will be greater than in the other, where only atmospheric pressure acts on the liquid. Under the force of this excess pressure, the liquid will begin to move. In the knee with compressed air, the liquid will fall, in the other it will rise. The liquid will come to equilibrium (stop) when the excess pressure of the compressed air is balanced by the pressure that the excess liquid column produces in the other leg of the pressure gauge.
The stronger the pressure on the film, the higher the excess liquid column, the greater its pressure. Consequently, the change in pressure can be judged by the height of this excess column.
The figure shows how such a pressure gauge can measure the pressure inside a liquid. The deeper the tube is immersed in the liquid, the greater the difference in the heights of the liquid columns in the manometer knees becomes., so, therefore, and fluid produces more pressure.
If you install the device box at some depth inside the liquid and turn it with a film up, sideways and down, then the pressure gauge readings will not change. That's the way it should be, because at the same level inside a liquid, the pressure is the same in all directions.

The picture shows metal manometer . The main part of such a pressure gauge is a metal tube bent into a pipe 1 , one end of which is closed. The other end of the tube with a tap 4 communicates with the vessel in which the pressure is measured. As pressure increases, the tube flexes. Movement of its closed end with a lever 5 and gears 3 passed to the shooter 2 moving around the scale of the instrument. When the pressure decreases, the tube, due to its elasticity, returns to its previous position, and the arrow returns to zero division of the scale.
Piston liquid pump.
In the experiment that we considered earlier (§ 40), it was found that water in a glass tube, under the action of atmospheric pressure, rose up behind the piston. This action is based piston pumps.
The pump is shown schematically in the figure. It consists of a cylinder, inside which goes up and down, tightly adhering to the walls of the vessel, the piston 1 . Valves are installed in the lower part of the cylinder and in the piston itself. 2 opening only upwards. When the piston moves upwards, water enters the pipe under the action of atmospheric pressure, lifts the bottom valve and moves behind the piston.
When the piston moves down, the water under the piston presses on the bottom valve, and it closes. At the same time, the pressure of the water opens the valve inside the piston, and the water flows into the space above the piston. With the next movement of the piston upwards, the water above it also rises in the place with it, which pours into the outlet pipe. At the same time, a new portion of water rises behind the piston, which, when the piston is subsequently lowered, will be above it, and this whole procedure is repeated again and again while the pump is running.
Hydraulic Press.

Pascal's law allows you to explain the action hydraulic machine (from Greek. hydraulicos- water). These are machines whose action is based on the laws of motion and equilibrium of liquids.
The main part of the hydraulic machine is two cylinders of different diameters, equipped with pistons and a connecting tube. The space under the pistons and the tube are filled with liquid (usually mineral oil). The heights of the liquid columns in both cylinders are the same as long as there are no forces acting on the pistons.
Let us now assume that the forces F 1 and F 2 - forces acting on the pistons, S 1 and S 2 - areas of pistons. The pressure under the first (small) piston is p 1 = F 1 / S 1 , and under the second (large) p 2 = F 2 / S 2. According to Pascal's law, the pressure of a fluid at rest is transmitted equally in all directions, i.e. p 1 = p 2 or F 1 / S 1 = F 2 / S 2 , from where:
F 2 / F 1 = S 2 / S 1 .
Therefore, the strength F 2 so much more power F 1 , How many times greater is the area of the large piston than the area of the small piston?. For example, if the area of the large piston is 500 cm 2, and the small one is 5 cm 2, and a force of 100 N acts on the small piston, then a force 100 times greater will act on the larger piston, that is, 10,000 N.
Thus, with the help of a hydraulic machine, it is possible to balance a large force with a small force.
Attitude F 1 / F 2 shows the gain in strength. For example, in the example above, the gain in force is 10,000 N / 100 N = 100.
The hydraulic machine used for pressing (squeezing) is called hydraulic press .

Hydraulic presses are used where a lot of power is required. For example, for squeezing oil from seeds at oil mills, for pressing plywood, cardboard, hay. In iron and steel works, hydraulic presses are used to make steel machine shafts, railway wheels, and many other products. Modern hydraulic presses can develop a force of tens and hundreds of millions of newtons.
The device of the hydraulic press is shown schematically in the figure. The body to be pressed 1 (A) is placed on a platform connected to a large piston 2 (B). The small piston 3 (D) creates a large pressure on the liquid. This pressure is transmitted to every point of the fluid filling the cylinders. Therefore, the same pressure acts on the second, large piston. But since the area of the 2nd (large) piston is larger than the area of the small one, then the force acting on it will be greater than the force acting on piston 3 (D). Under this force, piston 2 (B) will rise. When piston 2 (B) rises, the body (A) rests against the fixed upper platform and is compressed. The pressure gauge 4 (M) measures the fluid pressure. Safety valve 5 (P) automatically opens when the fluid pressure exceeds the allowable value.
From a small cylinder to a large liquid is pumped by repeated movements of the small piston 3 (D). This is done in the following way. When the small piston (D) is lifted, valve 6 (K) opens and liquid is sucked into the space under the piston. When the small piston is lowered under the action of liquid pressure, valve 6 (K) closes, and valve 7 (K") opens, and the liquid passes into a large vessel.
The action of water and gas on a body immersed in them.

Under water, we can easily lift a stone that can hardly be lifted in the air. If you submerge the cork under water and release it from your hands, it will float. How can these phenomena be explained?
We know (§ 38) that the liquid presses on the bottom and walls of the vessel. And if some solid body is placed inside the liquid, then it will also be subjected to pressure, like the walls of the vessel.
Consider the forces that act from the side of the liquid on the body immersed in it. To make it easier to reason, we choose a body that has the shape of a parallelepiped with bases parallel to the surface of the liquid (Fig.). The forces acting on the side faces of the body are equal in pairs and balance each other. Under the influence of these forces, the body is compressed. But the forces acting on the upper and lower faces of the body are not the same. On the upper face presses from above with force F 1 column of liquid tall h one . At the level of the lower face, the pressure produces a liquid column with a height h 2. This pressure, as we know (§ 37), is transmitted inside the liquid in all directions. Therefore, on the lower face of the body from the bottom up with a force F 2 presses a liquid column high h 2. But h 2 more h 1 , hence, the modulus of force F 2 more power modules F one . Therefore, the body is pushed out of the liquid with a force F vyt, equal to the difference of forces F 2 - F 1 , i.e.
|
|
But S·h = V, where V is the volume of the parallelepiped, and ρ W ·V = m W is the mass of fluid in the volume of the parallelepiped. Consequently, F vyt \u003d g m well \u003d P well, i.e. buoyant force is equal to the weight of the liquid in the volume of the body immersed in it(The buoyant force is equal to the weight of a liquid of the same volume as the volume of the body immersed in it). The existence of a force that pushes a body out of a liquid is easy to discover experimentally. On the image a shows a body suspended from a spring with an arrow pointer at the end. The arrow marks the tension of the spring on the tripod. When the body is released into the water, the spring contracts (Fig. b). The same contraction of the spring will be obtained if you act on the body from the bottom up with some force, for example, press it with your hand (raise it). Therefore, experience confirms that a force acting on a body in a fluid pushes the body out of the fluid. For gases, as we know, Pascal's law also applies. That's why bodies in the gas are subjected to a force pushing them out of the gas. Under the influence of this force, the balloons rise up. The existence of a force pushing a body out of a gas can also be observed experimentally. We hang a glass ball or a large flask closed with a cork to a shortened scale pan. The scales are balanced. Then a wide vessel is placed under the flask (or ball) so that it surrounds the entire flask. The vessel is filled with carbon dioxide, the density of which is greater than the density of air (therefore, carbon dioxide sinks down and fills the vessel, displacing air from it). In this case, the balance of the scales is disturbed. A cup with a suspended flask rises up (Fig.). A flask immersed in carbon dioxide experiences a greater buoyant force than that which acts on it in air. The force that pushes a body out of a liquid or gas is directed opposite to the force of gravity applied to this body. Therefore, prolcosmos). This explains why in the water we sometimes easily lift bodies that we can hardly keep in the air. A small bucket and a cylindrical body are suspended from the spring (Fig., a). The arrow on the tripod marks the extension of the spring. It shows the weight of the body in the air. Having lifted the body, a drain vessel is placed under it, filled with liquid to the level of the drain tube. After that, the body is completely immersed in the liquid (Fig., b). Wherein part of the liquid, the volume of which is equal to the volume of the body, is poured out from a pouring vessel into a glass. The spring contracts and the pointer of the spring rises to indicate the decrease in the weight of the body in the liquid. In this case, in addition to the force of gravity, another force acts on the body, pushing it out of the fluid. If the liquid from the glass is poured into the upper bucket (i.e., the one that was displaced by the body), then the spring pointer will return to its initial position (Fig., c).
Based on this experience, it can be concluded that the force that pushes a body completely immersed in a liquid is equal to the weight of the liquid in the volume of this body . We reached the same conclusion in § 48. If a similar experiment were done with a body immersed in some gas, it would show that the force pushing the body out of the gas is also equal to the weight of the gas taken in the volume of the body . The force that pushes a body out of a liquid or gas is called Archimedean force, in honor of the scientist Archimedes who first pointed to its existence and calculated its significance. So, experience has confirmed that the Archimedean (or buoyant) force is equal to the weight of the fluid in the volume of the body, i.e. F A = P f = g m and. The mass of liquid m f , displaced by the body, can be expressed in terms of its density ρ w and the volume of the body V t immersed in the liquid (since V l - the volume of the liquid displaced by the body is equal to V t - the volume of the body immersed in the liquid), i.e. m W = ρ W V t. Then we get: F A= g ρ and · V t Therefore, the Archimedean force depends on the density of the liquid in which the body is immersed, and on the volume of this body. But it does not depend, for example, on the density of the substance of a body immersed in a liquid, since this quantity is not included in the resulting formula. Let us now determine the weight of a body immersed in a liquid (or gas). Since the two forces acting on the body in this case are directed in opposite directions (gravity is down, and the Archimedean force is up), then the weight of the body in fluid P 1 will be less than the weight of the body in vacuum P = g m to the Archimedean force F A = g m w (where m w is the mass of liquid or gas displaced by the body). In this way, if a body is immersed in a liquid or gas, then it loses in its weight as much as the liquid or gas displaced by it weighs. Example. Determine the buoyancy force acting on a stone with a volume of 1.6 m 3 in sea water. Let's write down the condition of the problem and solve it. When the floating body reaches the surface of the liquid, then with its further upward movement, the Archimedean force will decrease. Why? But because the volume of the part of the body immersed in the liquid will decrease, and the Archimedean force is equal to the weight of the liquid in the volume of the part of the body immersed in it. When the Archimedean force becomes equal to the force of gravity, the body will stop and float on the surface of the liquid, partially immersed in it. The resulting conclusion is easy to verify experimentally. Pour water into the drain vessel up to the level of the drain pipe. After that, let's immerse the floating body into the vessel, having previously weighed it in the air. Having descended into the water, the body displaces a volume of water equal to the volume of the part of the body immersed in it. Having weighed this water, we find that its weight (Archimedean force) is equal to the force of gravity acting on a floating body, or the weight of this body in air. Having done the same experiments with any other bodies floating in different liquids - in water, alcohol, salt solution, you can make sure that if a body floats in a liquid, then the weight of the liquid displaced by it is equal to the weight of this body in air. It is easy to prove that if the density of a solid solid is greater than the density of a liquid, then the body sinks in such a liquid. A body with a lower density floats in this liquid. A piece of iron, for example, sinks in water but floats in mercury. The body, on the other hand, whose density is equal to the density of the liquid, remains in equilibrium inside the liquid. Ice floats on the surface of water because its density is less than that of water. The lower the density of the body compared to the density of the liquid, the smaller part of the body is immersed in the liquid . With equal densities of the body and liquid, the body floats inside the liquid at any depth. Two immiscible liquids, for example water and kerosene, are located in a vessel in accordance with their densities: in the lower part of the vessel - denser water (ρ = 1000 kg / m 3), on top - lighter kerosene (ρ = 800 kg / m 3) . The average density of living organisms inhabiting the aquatic environment differs little from the density of water, so their weight is almost completely balanced by the Archimedean force. Thanks to this, aquatic animals do not need such strong and massive skeletons as terrestrial ones. For the same reason, the trunks of aquatic plants are elastic. The swim bladder of a fish easily changes its volume. When the fish descends to a great depth with the help of muscles, and the water pressure on it increases, the bubble contracts, the volume of the fish's body decreases, and it does not push up, but swims in the depths. Thus, the fish can, within certain limits, regulate the depth of its dive. Whales regulate their diving depth by contracting and expanding their lung capacity. Sailing ships.Ships that sail on rivers, lakes, seas and oceans are built from different materials with different densities. The hull of ships is usually made of steel sheets. All internal fasteners that give ships strength are also made of metals. For the construction of ships, various materials are used, which, compared with water, have both higher and lower densities. How do ships float, take on board and carry large loads? An experiment with a floating body (§ 50) showed that the body displaces so much water with its underwater part that this water is equal in weight to the weight of the body in air. This is also true for any ship. The weight of water displaced by the underwater part of the ship is equal to the weight of the ship with cargo in the air or the force of gravity acting on the ship with cargo. The depth to which a ship is submerged in water is called draft . The deepest allowable draft is marked on the ship's hull with a red line called waterline (from Dutch. water- water). The weight of water displaced by the ship when submerged to the waterline, equal to the force of gravity acting on the ship with cargo, is called the displacement of the ship. At present, ships with a displacement of 5,000,000 kN (5 10 6 kN) and more are being built for the transportation of oil, i.e., having a mass of 500,000 tons (5 10 5 t) and more together with the cargo. If we subtract the weight of the ship itself from the displacement, then we get the carrying capacity of this ship. Carrying capacity shows the weight of the cargo carried by the vessel. Shipbuilding existed in Ancient Egypt, in Phoenicia (it is believed that the Phoenicians were one of the best shipbuilders), Ancient China. In Russia, shipbuilding originated at the turn of the 17th and 18th centuries. Mainly warships were built, but it was in Russia that the first icebreaker, ships with an internal combustion engine, and the nuclear icebreaker Arktika were built. Aeronautics.
Drawing describing the balloon of the Montgolfier brothers in 1783: "View and exact dimensions of the Balloon Globe, which was the first." 1786 Since ancient times, people have dreamed of being able to fly above the clouds, to swim in the ocean of air, as they sailed on the sea. For aeronautics At first, balloons were used, which were filled either with heated air, or with hydrogen or helium. In order for a balloon to rise into the air, it is necessary that the Archimedean force (buoyancy) F A, acting on the ball, was more than gravity F heavy, i.e. F A > F heavy As the ball rises, the Archimedean force acting on it decreases ( F A = gρV), since the density of the upper atmosphere is less than that of the Earth's surface. To rise higher, a special ballast (weight) is dropped from the ball and this lightens the ball. Eventually the ball reaches its maximum lift height. To lower the ball, part of the gas is released from its shell using a special valve. In the horizontal direction, the balloon moves only under the influence of the wind, so it is called balloon (from Greek air- air, stato- standing). Not so long ago, huge balloons were used to study the upper layers of the atmosphere, the stratosphere - stratostats . Before they learned how to build large aircraft for transporting passengers and cargo by air, controlled balloons were used - airships. They have an elongated shape, a gondola with an engine is suspended under the body, which drives the propeller. The balloon not only rises by itself, but can also lift some cargo: a cabin, people, instruments. Therefore, in order to find out what kind of load a balloon can lift, it is necessary to determine it. lifting force. Let, for example, a balloon with a volume of 40 m 3 filled with helium be launched into the air. The mass of helium filling the shell of the ball will be equal to: This means that this ball can lift a load weighing 520 N - 71 N = 449 N. This is its lifting force. A balloon of the same volume, but filled with hydrogen, can lift a load of 479 N. This means that its lifting force is greater than that of a balloon filled with helium. But still, helium is used more often, since it does not burn and is therefore safer. Hydrogen is a combustible gas. It is much easier to raise and lower a balloon filled with hot air. For this, a burner is located under the hole located in the lower part of the ball. Using a gas burner, you can control the temperature of the air inside the ball, which means its density and buoyancy. In order for the ball to rise higher, it is enough to heat the air in it more strongly, increasing the flame of the burner. When the burner flame decreases, the temperature of the air in the ball decreases, and the ball goes down. It is possible to choose such a temperature of the ball at which the weight of the ball and the cabin will be equal to the buoyancy force. Then the ball will hang in the air, and it will be easy to make observations from it. As science developed, there were also significant changes in aeronautical technology. It became possible to use new shells for balloons, which became durable, frost-resistant and light. Achievements in the field of radio engineering, electronics, automation made it possible to design unmanned balloons. These balloons are used to study air currents, for geographical and biomedical research in the lower layers of the atmosphere. Imagine an air-filled sealed cylinder with a piston mounted on top. If you start to put pressure on the piston, then the volume of air in the cylinder will begin to decrease, the air molecules will collide with each other and with the piston more and more intensively, and the pressure of compressed air on the piston will increase. If the piston is now abruptly released, then the compressed air will abruptly push it up. This will happen because with a constant piston area, the force acting on the piston from the compressed air will increase. The area of the piston remained unchanged, and the force from the side of the gas molecules increased, and the pressure increased accordingly. Or another example. A man stands on the ground, stands with both feet. In this position, a person is comfortable, he does not experience inconvenience. But what happens if this person decides to stand on one leg? He will bend one of his legs at the knee, and now he will lean on the ground with only one foot. In this position, a person will feel some discomfort, because the pressure on the foot has increased, and about 2 times. Why? Because the area through which gravity now presses a person to the ground has decreased by 2 times. Here is an example of what pressure is and how easy it is to detect in everyday life.
From the point of view of physics, pressure is a physical quantity numerically equal to the force acting perpendicular to the surface per unit area of this surface. Therefore, in order to determine the pressure at a certain point on the surface, the normal component of the force applied to the surface is divided by the area of the small surface element on which this force acts. And in order to determine the average pressure over the entire area, the normal component of the force acting on the surface must be divided by the total area of this surface. Pressure is measured in pascals (Pa). This pressure unit got its name in honor of the French mathematician, physicist and writer Blaise Pascal, the author of the basic law of hydrostatics - Pascal's Law, which states that the pressure exerted on a liquid or gas is transmitted to any point unchanged in all directions. For the first time, the unit of pressure "pascal" was put into circulation in France in 1961, according to the decree on units, three centuries after the death of the scientist.
One pascal is equal to the pressure exerted by a force of one newton, evenly distributed, and directed perpendicular to a surface of one square meter. In pascals, not only mechanical pressure (mechanical stress) is measured, but also the modulus of elasticity, Young's modulus, bulk modulus of elasticity, yield strength, proportionality limit, tear resistance, shear strength, sound pressure and osmotic pressure. Traditionally, it is in pascals that the most important mechanical characteristics of materials in the strength of materials are expressed. Atmosphere technical (at), physical (atm), kilogram-force per square centimeter (kgf / cm2) In addition to the pascal, other (off-system) units are also used to measure pressure. One such unit is the “atmosphere” (at). A pressure of one atmosphere is approximately equal to atmospheric pressure on the Earth's surface at sea level. Today, “atmosphere” is understood as the technical atmosphere (at).
The technical atmosphere (at) is the pressure produced by one kilogram-force (kgf) distributed evenly over an area of one square centimeter. And one kilogram-force, in turn, is equal to the force of gravity acting on a body with a mass of one kilogram under conditions of free fall acceleration equal to 9.80665 m/s2. One kilogram-force is thus equal to 9.80665 Newton, and 1 atmosphere turns out to be equal to exactly 98066.5 Pa. 1 at = 98066.5 Pa. In atmospheres, for example, the pressure in automobile tires is measured, for example, the recommended pressure in the tires of a GAZ-2217 passenger bus is 3 atmospheres. There is also the "physical atmosphere" (atm), defined as the pressure of a column of mercury, 760 mm high at its base, given that the density of mercury is 13595.04 kg / m3, at a temperature of 0 ° C and under conditions of a gravitational acceleration of 9, 80665 m/s2. So it turns out that 1 atm \u003d 1.033233 atm \u003d 101 325 Pa. As for the kilogram-force per square centimeter (kgf/cm2), this non-systemic unit of pressure is equal to normal atmospheric pressure with good accuracy, which is sometimes convenient for assessing various effects. The non-systemic unit "bar" is approximately equal to one atmosphere, but is more accurate - exactly 100,000 Pa. In the CGS system, 1 bar is equal to 1,000,000 dynes/cm2. Previously, the name "bar" was carried by the unit, now called "barium", and equal to 0.1 Pa or in the CGS system 1 barium \u003d 1 dyn / cm2. The word "bar", "barium" and "barometer" come from the same Greek word for "gravity".
Often, to measure atmospheric pressure in meteorology, the unit mbar (millibar), equal to 0.001 bar, is used. And to measure pressure on planets where the atmosphere is very rarefied - microbar (microbar), equal to 0.000001 bar. On technical pressure gauges, most often the scale has a graduation in bars. Millimeter of mercury column (mm Hg), millimeter of water column (mm of water column) The non-systemic unit of measure "millimeter of mercury" is 101325/760 = 133.3223684 Pa. It is designated "mm Hg", but sometimes it is designated "torr" - in honor of the Italian physicist, a student of Galileo, Evangelista Torricelli, the author of the concept of atmospheric pressure. The unit was formed in connection with a convenient way to measure atmospheric pressure with a barometer, in which the mercury column is in equilibrium under the action of atmospheric pressure. Mercury has a high density of about 13,600 kg/m3 and is characterized by low saturated vapor pressure at room temperature, which is why mercury was chosen for barometers at one time. At sea level, atmospheric pressure is approximately 760 mm Hg, it is this value that is now considered to be normal atmospheric pressure, equal to 101325 Pa or one physical atmosphere, 1 atm. That is, 1 millimeter of mercury is equal to 101325/760 pascals.
In millimeters of mercury, pressure is measured in medicine, meteorology, and aviation navigation. In medicine, blood pressure is measured in mmHg; in vacuum technology, it is graduated in mmHg, along with bars. Sometimes they even simply write 25 microns, meaning microns of mercury, when it comes to evacuation, and pressure measurements are carried out with vacuum gauges. In some cases, millimeters of water column are used, and then 13.59 mm of water column \u003d 1 mm Hg. Sometimes it is more expedient and convenient. A millimeter of a water column, like a millimeter of a mercury column, is an off-system unit, equal in turn to the hydrostatic pressure of 1 mm of a water column, which this column exerts on a flat base at a column water temperature of 4 ° C. If the piston is now abruptly released, then the compressed air will abruptly push it up. This will happen because with a constant piston area, the force acting on the piston from the compressed air will increase. The area of the piston remained unchanged, and the force from the side of the gas molecules increased, and the pressure increased accordingly. Or another example. A man stands on the ground, stands with both feet. In this position, a person is comfortable, he does not experience inconvenience. But what happens if this person decides to stand on one leg? He will bend one of his legs at the knee, and now he will lean on the ground with only one foot. In this position, a person will feel some discomfort, because the pressure on the foot has increased, and about 2 times. Why? Because the area through which gravity now presses a person to the ground has decreased by 2 times. Here is an example of what pressure is and how easy it is to detect in everyday life. pressure in physics From the point of view of physics, pressure is a physical quantity numerically equal to the force acting perpendicular to the surface per unit area of this surface. Therefore, in order to determine the pressure at a certain point on the surface, the normal component of the force applied to the surface is divided by the area of the small surface element on which this force acts. And in order to determine the average pressure over the entire area, the normal component of the force acting on the surface must be divided by the total area of this surface. The pressure in the SI system is measured in pascals (Pa). This pressure unit got its name in honor of the French mathematician, physicist and writer Blaise Pascal, the author of the basic law of hydrostatics - Pascal's Law, which states that the pressure exerted on a liquid or gas is transmitted to any point unchanged in all directions. For the first time, the unit of pressure "pascal" was put into circulation in France in 1961, according to the decree on units, three centuries after the death of the scientist. One pascal is equal to the pressure exerted by a force of one newton, evenly distributed, and directed perpendicular to a surface of one square meter. In pascals, not only mechanical pressure (mechanical stress) is measured, but also the modulus of elasticity, Young's modulus, bulk modulus of elasticity, yield strength, proportionality limit, tear resistance, shear strength, sound pressure and osmotic pressure. Traditionally, it is in pascals that the most important mechanical characteristics of materials in the strength of materials are expressed. Atmosphere technical (at), physical (atm), kilogram-force per square centimeter (kgf / cm2) In addition to the pascal, other (off-system) units are also used to measure pressure. One such unit is the “atmosphere” (at). A pressure of one atmosphere is approximately equal to atmospheric pressure on the Earth's surface at sea level. Today, “atmosphere” is understood as the technical atmosphere (at). The technical atmosphere (at) is the pressure produced by one kilogram-force (kgf) distributed evenly over an area of one square centimeter. And one kilogram-force, in turn, is equal to the force of gravity acting on a body with a mass of one kilogram under conditions of free fall acceleration equal to 9.80665 m/s2. One kilogram-force is thus equal to 9.80665 Newton, and 1 atmosphere turns out to be equal to exactly 98066.5 Pa. 1 at = 98066.5 Pa. In atmospheres, for example, the pressure in automobile tires is measured, for example, the recommended pressure in the tires of a GAZ-2217 passenger bus is 3 atmospheres. There is also the "physical atmosphere" (atm), defined as the pressure of a column of mercury, 760 mm high at its base, given that the density of mercury is 13595.04 kg / m3, at a temperature of 0 ° C and under conditions of a gravitational acceleration of 9, 80665 m/s2. So it turns out that 1 atm \u003d 1, at \u003d Pa. As for the kilogram-force per square centimeter (kgf/cm2), this non-systemic unit of pressure is equal to normal atmospheric pressure with good accuracy, which is sometimes convenient for assessing various effects. The non-systemic unit "bar" is equal to approximately one atmosphere, but is more accurate - exactly Pa. In the CGS system, 1 bar equal/cm2. Previously, the name "bar" was carried by the unit, now called "barium", and equal to 0.1 Pa or in the CGS system 1 barium \u003d 1 dyn / cm2. The word "bar", "barium" and "barometer" come from the same Greek word for "gravity". Often, to measure atmospheric pressure in meteorology, the unit mbar (millibar), equal to 0.001 bar, is used. And to measure pressure on planets where the atmosphere is very rarefied - microbar (microbar), equal to 0 bar. On technical pressure gauges, most often the scale has a graduation in bars. Millimeter of mercury column (mm Hg), millimeter of water column (mm of water column) The non-systemic unit of measurement "millimeter of mercury" is / 760 \u003d 133,Pa. It is designated "mm Hg", but sometimes it is designated "torr" - in honor of the Italian physicist, a student of Galileo, Evangelista Torricelli, the author of the concept of atmospheric pressure. The unit was formed in connection with a convenient way to measure atmospheric pressure with a barometer, in which the mercury column is in equilibrium under the influence of atmospheric pressure. Mercury has a high density of about kg / m3 and is characterized by a low saturated vapor pressure at room temperature, which is why mercury was chosen for barometers at one time. At sea level, atmospheric pressure is approximately 760 mm Hg, it is this value that is now considered to be normal atmospheric pressure, equal to Pa or one physical atmosphere, 1 atm. That is, 1 millimeter of mercury is equal to / 760 pascals. In millimeters of mercury, pressure is measured in medicine, meteorology, and aviation navigation. In medicine, blood pressure is measured in mm Hg; in vacuum technology, pressure measuring instruments are calibrated in mm Hg, along with bars. Sometimes they even simply write 25 microns, meaning microns of mercury, when it comes to evacuation, and pressure measurements are carried out with vacuum gauges. In some cases, millimeters of water column are used, and then 13.59 mm of water column \u003d 1 mm Hg. Sometimes it is more expedient and convenient. A millimeter of a water column, like a millimeter of a mercury column, is an off-system unit, equal in turn to the hydrostatic pressure of 1 mm of a water column, which this column exerts on a flat base at a column water temperature of 4 ° C. PressureThe force applied perpendicular to the surface of the body, under the action of which the body is deformed, is called the pressure force. Any force can act as a pressure force. This may be a force that presses one body against the surface of another, or the weight of a body acting on a support (Fig. 1). Rice. 1. Determination of pressure Pressure unitsIn the SI system, pressure is measured in pascals (Pa): 1 Pa = 1 N / m 2 The pressure does not depend on the orientation of the surface.
Obviously, depending on the surface area, the same pressure force can exert different pressure on this surface. This relationship is often used in technology to increase or, conversely, reduce pressure. The designs of tanks and tractors provide for reducing the pressure on the ground by increasing the area with the help of a caterpillar drive. The same principle underlies the design of skis: on skis, a person easily slides on the snow, however, having removed the skis, he immediately falls into the snow. The blade of cutting and piercing tools (knives, scissors, cutters, saws, needles, etc.) is specially sharpened: a sharp blade has a small area, so even a small force creates a lot of pressure, and it is easy to work with such a tool. Examples of problem solvingSurface area of the shovel that is in contact with the ground: where is the width of the blade, is the thickness of the cutting edge. Therefore, the pressure of the shovel on the ground: Let's convert the units to the SI system: blade width: cm m; incisal thickness mm m. Calculate: Pa MPa The pressure force in this case is the weight of the cube, so we can write: and the volume of the cube in turn: whence the edge of the cube: According to the tables, we determine the density of aluminum: kg / m. Copying materials from the site is possible only with permission portal administration and if there is an active link to the source. Pressure unitsInternational System of Units (SI)Pressure P is the physical quantity of force F acting on a unit surface area S, directed perpendicular to this surface. In the International System of Units (SI), pressure is measured in Pascals: Pa - Russian designation. 1 Pa = 1 Newton / 1 sq. meter (1 N/m²) For practical measurements in instrumentation and A, 1 Pa often turns out to be too small a pressure value, and for operating with real data, multiplying prefixes are used - (kilo, Mega), multiplying values by 1 thousand. and 1 million times respectively. 1 MPa = 1000 KPa = Pa Also, the scales of instruments for measuring pressure can be directly graduated in terms of Newton / meter, or their derivatives: Kilonewton, Meganewton / m², cm², mm². Then we get the following match: 1 MPa = 1 MN/m² = 1 N/mm² = 100 N/cm² = 1000 KN/m² = 1000 KPa = N/m² = Pa In Russia and Europe, the units Bar (Bar) and kg / m² (kgf / m²), as well as their derivatives (mBar, kg / cm²), are also widely used for measuring pressure. 1 Bar is a non-systemic unit equal to Pa. 1 kgf/cm² is a unit of pressure in the MKGSS system, and is widely used in industrial pressure measurements. 1 kgf / cm² \u003d kgf / m² \u003d 0. Bar \u003d 98066.5 Pa AtmosphereAtmosphere is a non-systemic unit of pressure measurement approximately equal to the atmospheric pressure of the Earth at the level of the World Ocean. There are two concepts of the atmosphere for measuring pressure:
In Russia, only the technical atmosphere is allowed for use in measurements, and according to some data, its validity period is limited to 2016. water columnA meter of water column is an off-system unit of pressure measurement used in a number of industries. Physically, it is equal to the pressure of a column of water 1 m high at a temperature of about 4 ° C and the standard gravitational acceleration for calibration is 9.80665 m / s². m of water. Art. - Russian designation. m H2O - international. The derived units are cm aq. Art. and mm w.c. Art. 1 m water Art. = 100 cm aq. Art. = 1000 mm w.c. Art. Relates to other pressure units as appropriate: 1 m water Art. = 1000 kg/m² = 0.Bar = 9.80665 Pa = 73.mmHg Art. mercury columnA millimeter of mercury is an off-system unit of pressure equal to 133.Pa. Synonym - Torr (Torr). mmHg Art. - Russian designation. mm Hg. - international. Use in Russia - not limited, but not recommended. It is used in a number of areas of technology. Ratio to water column: 1 mm Hg. Art. = 13.mm w.c. Art. US and UK unitsIn the United States and Britain, other pressure units are also used. This is due to the fact that lengths are expressed in feet and inches, and weights are in pounds, British and US tons. Examples of some of them:
Designation: in H2O. 1 in H2O = 249.08891 Pa. Designation: ft H2O. 1 ft H2O = 2989.Pa. Designation: in Hg. 1 in Hg = 3386.Pa. Designation: Psi. 1 Psi = 6894.Pa.
Designation: Psf. 1 Psf = 47.Pa. Designation: Tsi. 1 Tsi =.4 Pa. Designation: Tsf. 1 Tsf = 95760.3226 Pa. Designation: br.Tsi. 1 Tsi =.Pa. Designation: br.Tsf. 1 Tsf =.Pa. Pressure measuring instrumentsManometers, differential pressure gauges (pressure difference), vacuum gauges (vacuum measurement) are used to measure pressure. Pressure: pressure unitsTo understand what pressure is in physics, consider a simple and familiar example. Which? In a situation where we need to cut a sausage, we will use the sharpest object - a knife, and not a spoon, comb or finger. The answer is obvious - the knife is sharper, and all the force applied by us is distributed along the very thin edge of the knife, bringing the maximum effect in the form of separation of a part of the object, i.e. sausages. Another example - we are standing on loose snow. Legs fail, walking is extremely uncomfortable. Why, then, do skiers rush past us with ease and at high speed, without drowning and not getting entangled in the same loose snow? It is obvious that snow is the same for everyone, both for skiers and for walkers, but the effect on it is different. With approximately the same pressure, that is, weight, the surface area pressing on the snow varies greatly. The area of skis is much larger than the area of the sole of the shoe, and, accordingly, the weight is distributed over a larger surface. What helps or, on the contrary, prevents us from effectively influencing the surface? Why does a sharp knife cut bread better, and flat wide skis hold better on the surface, reducing penetration into the snow? In the seventh grade physics course, the concept of pressure is studied for this. pressure in physicsThe force applied to a surface is called pressure force. And pressure is a physical quantity that is equal to the ratio of the pressure force applied to a specific surface to the area of this surface. The formula for calculating pressure in physics is as follows: where p is pressure, F - pressure force, s is the surface area. We see how pressure is denoted in physics, and we also see that with the same force, the pressure is greater when the support area, or, in other words, the contact area of interacting bodies, is smaller. Conversely, as the area of support increases, the pressure decreases. That is why a sharper knife cuts any body better, and nails driven into a wall are made with sharp tips. And that is why skis hold on the snow much better than their absence. Pressure unitsThe unit of pressure is 1 newton per square meter - these are quantities already known to us from the seventh grade course. We can also convert pressure units N/m2 into pascals, units of measurement named after the French scientist Blaise Pascal, who developed the so-called Pascal's Law. 1 N/m = 1 Pa. In practice, other units of pressure are also used - millimeters of mercury, bars, and so on. Need help with your studies?All indecent comments will be deleted. What is the symbol for pressure in physics? Physics is a complex subject. Not everyone can understand it There are a lot of different interesting terms and formulas in physics. Useful information - pressure is measured in pascals As for the letter that stands for pressure in physics - the Latin letter P P,Pa nothing more to add, but the length of the message should be 40) Pressure is a physical quantity. It is defined as the force of pressure on any surface, to the area of this surface. Physical pressure is denoted by a small English letter p. The letter F stands for pressure force, and the letter S stands for surface area. The pressure is measured N / m2 (Newton per square meter). This value can be converted to Pascals (Pa). One Pa will be equal to one N / m. The answer to this easy question is from the field of physics, the initial course, which is taught in high school. From that time I distinctly remember that the letter for pressure, p. And the formula is p=f/s. This formula can be found in any physics textbook. As I remember from school physics lessons, pressure is denoted by the Latin letter p. I don't think anything has changed in a few years. Pressure is measured in pascals (indicated by Pa, or Pa in Latin letters). I also remember from physics lessons that pressure is measured in Pascals, and this unit is designated in the SI system as Pa. I think that such units of measurement do not change over time, since they were invented a long time ago and everyone uses them. Pressure is a physical quantity that characterizes the distribution of force over the area where it is applied. The ratio of this force F to the surface area S shows the pressure, which is written as a formula. In this formula, the Latin letter P denotes a physical quantity - pressure. Using the formula, you can follow the change in pressure. For example, in order to increase the pressure, you need to increase the force (value in the numerator) or decrease the application area (denominator). As correctly stated above, pressure in physics is denoted by the letter P. And the unit for measuring pressure in the International System of Units (SI) is indeed the pascal (Pa). This physical quantity owes its name to the most talented French scientist and writer of the 17th century Blaise Pascal, who in his short life (39 years) proved not only the existence of atmospheric pressure, but also carried out a huge amount of research and experiments. Pascal had a special weakness for mathematics, in the field of which he sometimes made discoveries during one night. Imagine that he is one of the creators of mathematical analysis, projective geometry, probability theory, and, among other things, the inventor of the first calculating machines - the prototype of modern computers! However, the most important thing is that fame and fortune did not harden the heart of a great man. Blaise Pascal, until the end of his days, took care of the common people, distributing most of the income to charity. Pascal's counting machine As far as I remember, pressure is denoted by the letter P. Moreover, you can use both large and small letters P. For example, here is the formula for excess gas pressure: The formula indicates 3 p are all different types of pressure. Letters near p indicate the type of pressure. In this case: pi is the excess pressure. The unit of measurement of this physical quantity (pressure) in the system of units is Pa (Pascal). This unit is named after the famous French. scientist and philosopher Blaise Pascal (years of life62). By the way, one of the programming languages Pascal is also named after him. In physics, the letter p (lower case) is used to denote pressure. The letter that shows the pressure looks like this: p. In the C system, pressure is measured in Pascals (Pa). What else can you say about pressure? Is that its physical definition, namely what it is. A represents this: the force acting on a unit surface located inside the body is pressure, and in the formula it looks like this p = F / S. It is the ratio of the force acting on a surface perpendicular to that surface to the area of that surface. The unit of pressure is measured in SI = 1Pa (Pascal). Top and bottom pressure: what does it meanWe all had our blood pressure taken. Almost everyone knows that the normal pressure is 120/80 mmHg. But not everyone can answer what these numbers actually mean. What do the numbers on the tonometer mean? Let's try to figure out what upper / lower pressure generally means, as well as how these values differ from each other. First, let's define the concepts. Pressure top and bottom: what does it mean?Blood pressure (BP) is one of the most important indicators, it demonstrates the functioning of the circulatory system. This indicator is formed with the participation of the heart, blood vessels and blood moving through them. Blood pressure is the pressure of blood on the wall of an artery Moreover, it depends on the resistance of the blood, its volume, "ejected" as a result of one contraction (this is called systole), and the intensity of contractions of the heart. The highest blood pressure can be observed when the heart contracts and "ejects" blood from the left ventricle, and the lowest - during entry into the right atrium, when the main muscle is relaxed (diastole). Here we come to the most important. Under the upper pressure or, in the language of science, systolic, refers to the pressure of the blood during contraction. This indicator shows how the heart contracts. The formation of such pressure is carried out with the participation of large arteries (for example, the aorta), and this indicator depends on a number of key factors.
The ratio of pressure in the human body As for the lower pressure (in other words, diastolic), it shows what resistance the blood experiences while moving through the blood vessels. Lower pressure occurs when the aortic valve closes and blood cannot return to the heart. In this case, the heart itself is filled with other blood, saturated with oxygen, and prepares for the next contraction. The movement of blood occurs as if by gravity, passively. Factors that affect diastolic pressure include: Note! In the normal state, the difference between the two indicators ranges between 30 mm and 40 mm Hg, although much here depends on the person's well-being. Despite the fact that there are specific figures and facts, each organism is individual, as well as its blood pressure. We conclude: in the example given at the beginning of the article (120/80), 120 is an indicator of upper blood pressure, and 80 is lower. Blood pressure - norm and deviations Tellingly, the formation of blood pressure depends mainly on lifestyle, nutritious diet, habits (including bad ones), and the frequency of stress. For example, by eating a particular food, you can specifically lower / increase blood pressure. It is authentically known that there were cases when people were completely cured of hypertension after changing their habits and lifestyle. Why do you need to know the value of blood pressure?For every 10 mmHg increase, the risk of cardiovascular disease increases by about 30 percent. People with high blood pressure are seven times more likely to develop a stroke, four times more likely to have coronary heart disease, and two times more likely to develop damage to the blood vessels of the lower extremities. It is important to know your blood pressure That is why finding out the cause of symptoms such as dizziness, migraines or general weakness should begin with measuring blood pressure. In some cases, the pressure must be constantly monitored and checked every few hours. Why is it important to know your blood pressure? How pressure is measuredBlood pressure measurement In most cases, blood pressure is measured using a special device consisting of the following elements:
The cuff is placed over the shoulder. During the measurement process, it is necessary to adhere to certain requirements, otherwise the result may be incorrect (underestimated or overestimated), which, in turn, may affect the subsequent treatment tactics. Blood pressure - measurement
Rules for using a mechanical tonometer How to use a semi-automatic tonometer Common mistakes in measuring blood pressure Note! If a person has a heart rhythm disorder, then measuring blood pressure will be a more complicated procedure. Therefore, it is better that a medical officer does this. How to evaluate your blood pressureThe higher a person's blood pressure, the greater the likelihood of such ailments as stroke, ischemia, renal failure, and so on. For self-assessment of the pressure indicator, you can use a special classification developed back in 1999. Table number 1. Assessment of the level of blood pressure. Norm * - optimal in terms of the development of vascular and heart diseases, as well as mortality. Note! If the upper and lower blood pressure are in different categories, then the one that is higher is selected. Table number 2. Assessment of the level of blood pressure. Hypertension Norms of blood pressure in adults Normal pressure settings Average values of maximum and minimum blood pressure for students blood pressure in babies Drawing conclusionsChanges in blood pressure So, blood pressure is the pressure that is exerted on the walls of blood vessels. Under the upper blood pressure is meant the indicator during the maximum contraction of the heart muscle, and under the lower - during relaxation. There are many factors that affect both indicators, but habits, nutrition and lifestyle are considered the main ones. An increase / decrease in blood pressure can indicate the development of many serious diseases, which is why it is so important to periodically measure and be able to evaluate the results. Hypertension and hypotension Let's do an experiment. Let us take a small board with four nails driven into the corners and place it with the points up on the sand. We put a weight on top of it (Fig. 81). We will see that the nail heads are only slightly pressed into the sand. If we turn the board over and put it again (together with the weight) on the sand, now the nails will go into it much deeper (Fig. 82). In both cases, the weight of the board was the same, but the effect was different. Why? The whole difference in the cases under consideration was that the surface area on which the nails rested was larger in one case and smaller in the other. After all, at first the heads of the nails touched the sand, and then their points. We see that the result of the impact depends not only on the force with which the body presses on the surface, but also on the area of this surface. It is for this reason that a person who is able to slide on loose snow on skis immediately falls into it as soon as he takes them off (Fig. 83). The force applied perpendicular to the surface is called force of pressure to this surface. Pressure force should not be confused with pressure. Pressure- this is a physical quantity equal to the ratio of the pressure force applied to a given surface to the area of \u200b\u200bthis surface: p - pressure, F - pressure force, S - area. So, to determine the pressure, it is necessary to divide the pressure force by the surface area on which the pressure is applied. With the same force, the pressure is greater when the area of support is smaller, and, conversely, the larger the area of support, the less pressure. In cases where the pressure force is the weight of the body on the surface (F = P = mg), the pressure exerted by the body can be found by the formula If the pressure p and the area S are known, then the pressure force F can be determined; To do this, you need to multiply the pressure by the area: F = pS (32.2) The pressure force (like any other force) is measured in newtons. Pressure is measured in pascals. Pascal(1 Pa) is the pressure that a pressure force of 1 N produces when applied to a surface of 1 m 2: 1 Pa \u003d 1 N / m 2. Other pressure units are also used - hectopascal (hPa) and kilopascal (kPa): 1 hPa = 100 Pa, 1 kPa = 1000 Pa. 1. Give examples showing that the result of the action of a force depends on the area of \u200b\u200bthe support on which this force acts. 2. Why doesn't a skier fall into the snow? 3. Why does a sharp button go into wood more easily than a blunt one? 4. What is called pressure? 5. What units of pressure do you know? 6. What is the difference between pressure and pressure force? 7. How can you find the pressure force, knowing the pressure and the surface area to which the force is applied? PHYSICS. 1. The subject and structure of physics F. the science that studies the simplest and at the same time the most. general properties and laws of motion of the objects of the material world surrounding us. As a result of this generality, there are no natural phenomena that do not have physical. properties... Physical Encyclopedia A science that studies the simplest and at the same time the most general patterns of natural phenomena, the principles and structure of matter and the laws of its motion. The concepts of F. and its laws underlie all natural science. F. belongs to the exact sciences and studies quantities ... Physical Encyclopedia PHYSICS- PHYSICS, a science that studies, together with chemistry, the general laws of the transformation of energy and matter. Both sciences are based on two basic laws of natural science - the law of conservation of mass (the law of Lomonosov, Lavoisier) and the law of conservation of energy (R. Mayer, Jaul ... ... Big Medical Encyclopedia Stellar physics is one of the branches of astrophysics that studies the physical side of stars (mass, density, ...). Contents 1 Dimensions, masses, density, luminosity of stars 1.1 Mass of stars ... Wikipedia I. The subject and structure of physics Physics is a science that studies the simplest and, at the same time, the most general patterns of natural phenomena, the properties and structure of matter, and the laws of its motion. Therefore, the concepts of F. and its laws underlie everything ... ... In a broad sense, pressure in excess of atmospheric pressure; in specific technical and scientific tasks, pressure exceeding the value characteristic of each task. Equally conventionally found in the literature is the subdivision of D. century. to high and ... ... Great Soviet Encyclopedia - (from ancient Greek physis nature). The ancients called physics any study of the surrounding world and natural phenomena. This understanding of the term physics was preserved until the end of the 17th century. Later, a number of special disciplines appeared: chemistry, which studies the properties of ... ... Collier Encyclopedia Investigation of the influence exerted on matter by very high pressures, as well as the creation of methods for obtaining and measuring such pressures. The history of the development of high pressure physics is an amazing example of an unusually rapid progress in science, ... ... Collier Encyclopedia Solid state physics is a branch of condensed matter physics whose task is to describe the physical properties of solids from the point of view of their atomic structure. It developed intensively in the 20th century after the discovery of quantum mechanics. ... ... Wikipedia Contents 1 Preparation methods 1.1 Evaporation of liquids ... Wikipedia Books
|













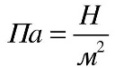



 But it's not just the area. The magnitude of the applied force also plays an important role. If, for example, on the same. board (see Fig. 81) put another weight, then the nails (with the same area of \u200b\u200bsupport) will sink even deeper into the sand.
But it's not just the area. The magnitude of the applied force also plays an important role. If, for example, on the same. board (see Fig. 81) put another weight, then the nails (with the same area of \u200b\u200bsupport) will sink even deeper into the sand.