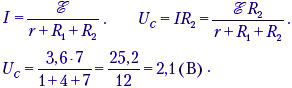Sources: Firefly, rotting wood, deep fish. Completed by: Student 11 "B" class Alexander Vavilkin. properties of x-rays. Radiation occurs due to an increase in internal energy radiating body. Application: Road signs, lighting engineering. Sources: Glowing paint. Sources: sun, ultraviolet lamps. The glow occurs without a change in body temperature. Wilhelm Conrad Roentgen. During operation, strongly penetrating radiation occurs. Application: Drying, home heating, etc.
"Magnetic field force Ampere" - Feature magnetic field. where B is the magnetic induction, F is the force, I is the current, ?l is the length of the conductor. A magnetic field. Ampere power. Determine the direction of the force acting on the conductor with current from the magnetic field. Modulus of magnetic induction vector. Grade 11. The direction of the magnetic induction vector. Determine the direction of the current in a conductor in a magnetic field. Ampere's law.
"Radar location in physics" - MOU "Gymnasium No. 1". Theoretical part. Weak signals are amplified in the amplifier and sent to the indicator. Years pass, the emerging exotic technique turns into an ordinary, widely used one. Tasks: Purpose: Relevance: Hypothesis: Reflected impulses propagate in all directions. - Radar - detection and exact location of an invisible target. The radiation is carried out by short pulses with a duration of 10-6 s.
"Mechanical vibrations class 11" - Conditions for the occurrence of a wave: Sounds of even the same tone can be of different loudness. The loudness of sound is related to the energy of oscillations in the source and in the wave. Wave characteristics: Occur in any medium (liquids, gases, solids). Occur only in solids. sound waves. Waves on the surface of a liquid are neither longitudinal nor transverse. The energy of oscillations is determined by the amplitude of oscillations. Waves are: 2. Longitudinal - in which oscillations occur along the direction of wave propagation.
"The binding energy of atomic nuclei" - Task: 1. Physics, grade 11 Lesson number 12. Bond energy atomic nuclei. Conventional unit: (UE-0) -3 min. Group work. Technology: modular lesson: combined.
"Theme Types of radiation" - Infrared - "thermal" radiation. Fluorescent lamps Quartz instrument in the solarium laboratory. X-ray photograph (roentgenogram) of his wife's hand, taken by V. K. Roentgen. Ufi-. Completed by a student of 11 "B" class Ekaterina Dvigalova. Infrared radiation Ultraviolet radiation x-ray radiation. UV sources. William Hyde Wollaston (English) 1801. Application of RI. Wilhelm Conrad Roentgen 1895. Ionizes the air. Ultraviolet radiation.
Part 3
Tasks С1–С6 are the tasks complete solution which must be recorded in the answer sheet No. 2. It is recommended to make a preliminary decision on a draft. When making a decision in the answer form No. 2, first write down the task number ( C1 etc.), and then solving the corresponding problem.
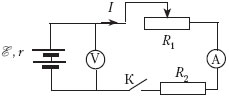
C1. The photo shows an electrical circuit consisting of a resistor, a rheostat, a key, a digital voltmeter connected to a battery, and an ammeter. Make a circuit diagram of this circuit, and using the laws direct current, explain how the current in the circuit and the voltage on the battery will change (increase or decrease) when the rheostat slider is moved to the extreme right position.
Complete the right decision each task С2–С6 should include laws and formulas, the application of which is necessary and sufficient to solve the problem, as well as mathematical transformations, calculations with a numerical answer, and (if necessary) a figure explaining the solution.
C2. starting speed of a projectile fired from a cannon vertically upwards is 500 m/s. At the point of maximum rise, the projectile exploded into two fragments. The first fell to the ground near the point of the shot, having a speed 2 times greater than the initial velocity of the projectile, and the second in the same place - 100 s after the break. What is the ratio of the mass of the first fragment to the mass of the second fragment? Ignore air resistance.
 C3.
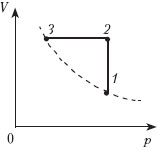
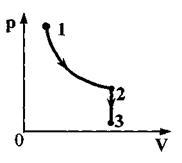
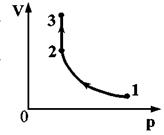
One mole of perfect monatomic gas first heated and then cooled to the initial temperature of 300 K, reducing the pressure by 3 times (see Fig.). How much heat is reported to the gas in the area 1–2
?
C3.
One mole of perfect monatomic gas first heated and then cooled to the initial temperature of 300 K, reducing the pressure by 3 times (see Fig.). How much heat is reported to the gas in the area 1–2
?
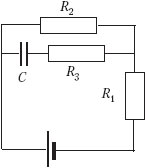 C4.
A 2 uF capacitor is connected to a DC source with an EMF of 3.6 V and an internal resistance of 1 ohm. Resistors R 1 = 4 ohm, R 2 = 7 ohm, R 3 = 3 ohms. What is the charge on the left side of the capacitor?
C4.
A 2 uF capacitor is connected to a DC source with an EMF of 3.6 V and an internal resistance of 1 ohm. Resistors R 1 = 4 ohm, R 2 = 7 ohm, R 3 = 3 ohms. What is the charge on the left side of the capacitor?
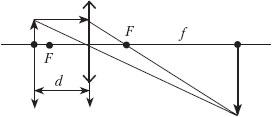
C5. An image of a rod with a fivefold magnification was obtained on the screen using a thin lens. The rod is located perpendicular to the main optical axis, the screen plane is also perpendicular to this axis. The screen was moved 30 cm along the main optical axis of the lens. Then, with the lens position unchanged, the rod was moved so that the image became sharp again. In this case, an image with a threefold increase was obtained. Determine focal length lenses.
C6. A drug with an activity of 1.7 · 10 11 particles per second is placed in a copper container weighing 0.5 kg. By how much did the temperature of the container rise in 1 hour, if it is known that this radioactive substance emits α-particles with an energy of 5.3 MeV? Assume that the energy of all α-particles is completely converted into the internal energy of the container. The heat capacity of the drug and heat exchange with environment neglect.
Instructions for checking and evaluating works, part 3.
Task solutions С1–С6 part 3 (with a detailed answer) are evaluated by an expert commission. Based on the criteria presented in the tables below, for the completion of each task, depending on the completeness and correctness of the answer given by the student, from 0 to 3 points are assigned.
A task C1
1) Equivalent electrical circuit circuit, taking into account internal resistance batteries, shown in the figure, where I is the current in the circuit.
|
|
The current through the voltmeter practically does not flow, and the resistance of the ammeter is negligible. 2. The current strength in the circuit is determined by Ohm's law for a closed (complete) circuit: In accordance with Ohm's law for a circuit section, the voltage measured by a voltmeter is U=I(R 1 + R 2) = – Ir. 3. When moving the rheostat slider to the right, its resistance decreases, which leads to a decrease in the total resistance of the circuit. At the same time, the current in the circuit increases, and the voltage on the battery decreases. |
|
- correctly stated physical phenomena and laws (in this case – Ohm's law for a section of a circuit and for a closed circuit) and the correct answer is given; - Reasoning leading to the correct answer is given. |
|
Correct solution presented and correct answer received, but: – not all physical phenomena or laws necessary for a complete correct answer are indicated; – electrical diagram not shown - Reasoning leading to the answer is not presented. |
|
Physical phenomena or laws are correctly indicated, but the reasoning contains an error that led to an incorrect answer. - Contains only the correct indication of physical phenomena or laws. – Only the correct scheme is presented electrical circuit. - Only the correct answer is presented. |
|
All decision cases that do not meet the above criteria for scoring 1, 2, 3 points. |
|
A task C2
Sample possible solution |
|
According to the law of conservation of energy, the height of the projectile can be calculated by the formula:
|
|
From the law of conservation of energy, we determine the initial velocity of the first fragment: The initial speed of the second fragment after the projectile burst can be determined by the formula: where t is the flight time of the second fragment. According to the law of conservation of momentum, |
|
Criteria for assessing the performance of the assignment |
|
A complete correct solution is given, including the following elements: 1) the formulas expressing physical laws, laws of conservation of energy and momentum, kinematics formula); |
|
The solution contains an error in the necessary mathematical transformations and lacks any numerical calculations. |
|
A task C3
Example of a possible solution |
|
According to the first law of thermodynamics , Q 12 = ∆U 12 + A 12 , where ∆U 12 = 3/2v R(T 2 - T 1);A 12 = ν R(T 2 - T 1). |
|
Consequently, Q 12 = 5/2v R(T 2 - T 1). According to Charles' law, Therefore, T 2 = 3T 1 and Q 12 = 5v RT 1 . Answer: Q 12 ≈ 12.5 kJ . |
|
A complete correct solution is given, including the following elements: the application of which is necessary to solve the problem in the chosen way (in this decision – first law of thermodynamics, formulas for calculating the change in internal energy and work of a gas, Charles's law); 2) the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed. |
|
The correct solution is presented only in general view, without any numerical calculations. - spelled correctly necessary formulas, the correct answer is written, but the transformations leading to the answer are not presented. – An error was made in mathematical transformations or calculations that led to an incorrect answer. |
|
The solution contains an error in necessary mathematical transformations and there are no numerical calculations. - All recorded initial formulas necessary to solve the problem, but ONE of them has a mistake. – Missing one of the formulas required to solve the problem. |
|
All cases of a solution that do not meet the above criteria for scoring 1, 2, 3 points (use of an inapplicable law, the absence of more than one source equation, scattered entries, etc.). |
|
A task C4
Example of a possible solution |
After the capacitor is charged, the current through the resistor R 3: I 3 = 0 ⇒ U 3 = 0 ⇒ U R 3 C = U 3 + U C = U C . At parallel connection U 2 =U R 3 C = U C = U C . |
q=CU C. q = 2 10 -6 2.1 = 4.2 10 -6 (C). Answer: q= 4.2 μC. |
Criteria for evaluating task completion by 3 points |
1) the formulas expressing physical laws are correctly written down, the application of which is necessary to solve the problem in the chosen way (in this solution - Ohm's laws for the plot and complete chain, connection of the charge of the capacitor with the voltage on its plates, equality of voltages in parallel connection) |
A task C5
Example of a possible solution (drawing required) |
The figure schematically shows the position of the lens, the object and the image on the screen formed by the rays passing through the lens.
|
Using the thin lens formula where d is the distance from the lens to the object, f- the distance from the lens to the screen, we determine the focal length of the lens. As follows from the similarity of triangles (see figure), the increase G given by the lens is determined by the ratio f/d= 5, which allows you to record the focal length of the lens
After moving the screen a distance l = 0.3, for the new position of the object and image, you can write an expression for the focal length: where is the magnification given by the lens after moving the screen. Here f 1 = f–l is the distance from the lens to the screen, and d 1 - distance from the lens to the object after moving the screen. Excluding f from equations (1) and (2), we obtain the focal length of the lens: Answer: F= 0.15 m, or 15 cm. |
Criteria for evaluating task completion by 3 points |
1) the formulas expressing physical laws are correctly written down, the application of which is necessary to solve the problem in the chosen way (in this solution - lens formulas and magnification given by the lens) <...> |
A task C6
Example of a possible solution |
During the time ∆ t amount of heat released in the preparation Q = A∙ε∙∆ t, where BUT is the drug activity, ε is the energy of the α-particle, ∆ t- time. The change in container temperature is determined by the equality Q = cm∆T, where With – specific heat copper, m– mass of the container, ∆ T– change in container temperature. The released amount of heat is used to heat the container. From here Answer. ∆T≈ 2.7 K. |
Criteria for evaluating task completion by 3 points |
1) the formulas expressing physical laws are correctly written down, the application of which is necessary to solve the problem in the chosen way (in this solution - the formula for the energy released by the drug and the formula for calculating the amount of heat received by the container when heated) <...> |
Text in brackets< ... >evaluation criteria for 3 points, as well as evaluation criteria for 2, 1 and 0 points are the same as in the previous task. - Ed.
Authors-compilers M.Yu. Demidova, V.A. mushrooms and others presented examination option 2009, modified to 2010 requirements. See No. 3/2009 for work instructions and any references you may need. - Ed.
Task 26.Thermally insulated vessel V= 2 m3 divided by porous partition into two equal parts. AT initial moment in one part the vessel is located m= 1 kg of helium, and in the other - m= 1 kg of argon, and the average the quadratic velocity of argon and helium atoms is the same and is 1000 m/s. Helium atoms can freely penetrate through the pores in partition, while argon atoms do not. Determine the temperature of the helium argon mixture after equilibrium has been established in the system.
Key elements of the solution
1. After equilibrium is established in the system, the temperature of bothparts of the vessel will become the same and equal to T, and heliumdistributed evenly throughout the vessel.
2. The temperature in the vessel is determined from the conservation law energy:
ε = 2= (νHe + νAr ) RT , where νHe = and νAr = is the number of moles of helium and argon.
Hence T = 2
3. Substituting numerical data, we get: T = 292 K.
Task 27.VesselvolumeV= 2 m3 is divided by a porous partition into two equal parts. At the initial moment, in one part of the vessel there is m= 1 kg of helium, and in another m= 1 kg of argon. Initial helium temperature equal to the temperature of argon T = 300 K. Helium atoms can freely penetrate through the septum, but argon atoms do not. Determine internal energy of the gas remaining in that part of the vessel where helium was originally located after equilibrium was established in system.
 Answer:ε
=
ν
1
font-size: 13.0pt;color:black;letter-spacing:-.6pt">=
mRT= 467 kJ.
Answer:ε
=
ν
1
font-size: 13.0pt;color:black;letter-spacing:-.6pt">=
mRT= 467 kJ.
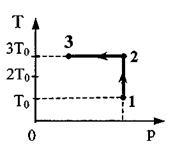
Task 28.One mole of argon performs the process 1-2-3. In section 2 - 3, 300 J of heat is supplied to the gas (see figure). T0 \u003d 10 K. Find the ratio of the work done by the gas during the entire process A 123 to the corresponding total amount of heat supplied to it Q123 .
Key elements of the solution
1. Write down the formula for calculating the work done during the entire
process: A123 = A12 + A2z. 2. Write down the formula for calculating work AJ 2 = νRΔT 12 or considering thatΔT 12 \u003d 2T0 A12 \u003d 2 νRT 0 . Write down the first law of thermodynamics for section 2-3 Q 23 = ∆U 23 + A23. Note that in an isothermal processΔU 23 = 0. Then Q 23 = A23 and A123 = 2 νRT 0 + Q 23. Write down the first law of thermodynamics for section 1-2 Q 12 \u003d ΔU 12 + A 12. 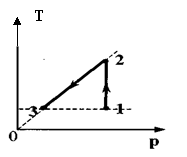 Write down the formula for calculating the change in internal energyΔU 12 = EN-US" style="font-size: 13.0pt;color:black">νRΔT 12 or considering thatΔT 12 = 2T0: ΔU 12 = 3 νRT 0 . 6. After the transformation Q 12 = 5 νRT 0 , Q 123 = 5 νRT 0 + Q 23 the desired ratio is font-size:13.0pt; color:black"> Problem 29.
One mole of an ideal monatomic gas was first heated and then cooled to an initial temperature of 300 K, reducing the pressure by a factor of 3 (see figure). How much heat is reported to the gas in section 1-2?
Write down the formula for calculating the change in internal energyΔU 12 = EN-US" style="font-size: 13.0pt;color:black">νRΔT 12 or considering thatΔT 12 = 2T0: ΔU 12 = 3 νRT 0 . 6. After the transformation Q 12 = 5 νRT 0 , Q 123 = 5 νRT 0 + Q 23 the desired ratio is font-size:13.0pt; color:black"> Problem 29.
One mole of an ideal monatomic gas was first heated and then cooled to an initial temperature of 300 K, reducing the pressure by a factor of 3 (see figure). How much heat is reported to the gas in section 1-2?
Answer: 5vRT=12.5 kJ
 Task 30.One mole of an ideal monatomic gas first expanded isothermally (
T1
= 300 K).Then the gas was cooled by lowering the pressure by 3 times
(see picture). How much heat is given off
gas in section 2-3?
Task 30.One mole of an ideal monatomic gas first expanded isothermally (
T1
= 300 K).Then the gas was cooled by lowering the pressure by 3 times
(see picture). How much heat is given off
gas in section 2-3?
Key elements of the solution
1. Write down the first law of thermodynamicsΔ U = Q + Avn. With.
Please note that in section 2-3: A2z \u003d 0. Then Q 23 \u003d ΔU 23.
2. Write down the formula for calculating the change in internal energy:ΔU 23 = νR (Tz - T2). Note that T2 = T1.
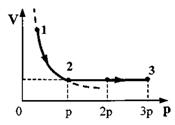 3.
Apply Charles' law for states 2 and 3: = and get the ratio T3 = . 4. Substituting the obtained value of T3 into the formulaΔU 23 \u003d νR (Tz - T2) \u003d Q 23 , calculate the amount of heat: Q 23 \u003d - ν RT 1 \u003d 2.5 kJ.
3.
Apply Charles' law for states 2 and 3: = and get the ratio T3 = . 4. Substituting the obtained value of T3 into the formulaΔU 23 \u003d νR (Tz - T2) \u003d Q 23 , calculate the amount of heat: Q 23 \u003d - ν RT 1 \u003d 2.5 kJ.
Task 31.One mole of an ideal monatomic gas first expanded isothermally ( T1 = 300 K). Then the gas was heated isobarically, increasing the temperature by 1.5 times (see figure). How much heat received gas in section 2-3?
Key elements of the solution
1.Write down the first law of thermodynamics for isobaric extensions: Q 23 \u003d ∆ U 23 + A23. 2. Write down the formulas for calculating the change in internal energy and gas work:
Δ U 23 = v R(T 3 - T2). A23 = v R(T 3 - T2). Note that T2 = T1 and T3 = 1.5T2.
3. Carry out transformations and get the formula for calculating the amount of heat and its numerical value: Q 23 \u003d 1.25 ν RT 1 = 3.1 kJ.
Task 32.1 mole of an ideal monatomic gas first isothermally compressed ( T1 =300 K). Then gasheated, increasing the pressure by 3 times (see Fig. picture). How much heat received gas in section 2-3?
Answer:Q23 = 3 ν RT 1 = 7.5 kJ.
Task 33. One mole of helium completes the cycle shown in pV-diagram (see figure). Plot 1-2-adiabatic, 2-3 - isotherm, 3-1 - isobar. The work done on the gas per cycle is equal to A. In section 2-3, the gas gives off the amount of heat Q. What is the temperature difference between states 1 and 2?
Key elements of the solution
1. Write down the expression for the gas work per cycle:
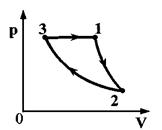 A \u003d A 12 + A23 + A 31 or work of helium at isothermalprocess A23 = A - A 12 - A31
A \u003d A 12 + A23 + A 31 or work of helium at isothermalprocess A23 = A - A 12 - A31
2. Write down what work:
in an adiabatic process is equal to A 12 = ∆ U= ν RΔT;
in isothermal process equal to A23 \u003d - Q;
at isobaric process is equal to A 31 = v RΔT.
3. Substituting all the obtained values of work for separate sections into the formula for the work of gas per cycle, get - Q=A- EN-US style="font-size:13.0pt;color:black"">νR ΔT - ν RΔT, where do you expressΔ Tand write the correct answer:Δ T=http://pandia.ru/text/79/312/images/image067.jpg" align="left" width="201 height=127" height="127"> Task 34.State of a monatomic ideal gas changes in two cycles: 1421 and 1231, shown in the figure on pV-diagram.Whatis equal to the efficiency ratio of heat engines based on using these cycles? based on the using these cycles?
Answer:Task 36.The state of an ideal gas changes in a closed cycle. From state 1 with temperature T1 = 1900 K gas, expanding adiabatically, goes to state 2 with temperature T2 = 1260 K. From state 2 the gas passes to state 3 with temperature T3 = 360 K byisochoric cooling. The gas is transferred from state 3 to state 4 withtemperature T4 = 540 K by adiabatic compression, from state 4 -to state 1 by isochoric heating. Calculate the efficiency for this cycle . Answer: η ≈ 0.34
Problem 37. A vessel with a small crack contains a monatomic ideal gas. In the experiment, the gas pressure and its volume decreased by a factor of three. How many times has it changed internal energy gas in a vessel?
Key elements of the solution
1. Write down the Clapeyron - Mendeleev equation for two states: p 1 V 1 = ν 1 RT and p 2 V 2 = ν 2 RT . 2. Write down that the internal energy of an ideal gas directly proportional to the amount of substance andabsolute temperature U~vT. 3. Analyze these expressions taking into account the condition tasks and get the correct answer: .